16. November 2022 No Comment
Please refer to the appropriate style manual or other sources if you have any questions. Furthermore, since all subsequent procedural steps are dependent on that initial valence electron count,all elements in the same group will gain or lose the same number of electrons to achieve an octet configuration. Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Additionally, both the nitrate ion and the sulfite ion contain three oxygens, but these polyatomic ions do not share a common suffix. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Lithium Hydrogen Sulfate. Unfortunately, these processes were quite lengthy. Finally, combine the two ions to form an electrically neutral compound. The formula for the barium ion is Ba2+ .  Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. After all, both liquids have ionic compounds dissolved in them. Finally, combine the two ions to form an electrically neutral compound.
Webbarium (Ba), chemical element, one of the alkaline-earth metals of Group 2 (IIa) of the periodic table. After all, both liquids have ionic compounds dissolved in them. Finally, combine the two ions to form an electrically neutral compound.
WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. Therefore, the resultant ion is symbolized asI1and is named the iodide ion. Created by Sal Khan. Finally, a new ion name was presented.
The barium ion (Ba2+ B a 2 + ) and sulfide ion (S2 ) can combine together to form barium sulfide, which has a formula of What is the formula for an ionic compound made of barium? This difference is significant because the hydrogen carbonate ion and some related ions have a crucial role in controlling the acid-base properties of blood.
118 Names and Symbols of the Periodic Table Quiz, https://www.britannica.com/science/barium, barium - Student Encyclopedia (Ages 11 and up). Write the chemical formula for the ionic compound formed by each pair of ions. The formula for an ionic compound follows several conventions. Oxygen is in Group 16, and tends to form O2 ions as its oxide. provide the ion name for the resultant ion. Both phosphorus and chlorine are nonmetals. For each pair of elements, determine the charge for their ions and write the proper formula for the resulting ionic compound between them. Nitrogen is in Group 15, and tends to form #N^(3-)# ions as its nitride. The element is used in metallurgy, and its compounds are used in pyrotechnics, petroleum production, and radiology. [21], Rubidium was discovered in 1861 by Robert Bunsen and Gustav Kirchhoff, in Heidelberg, Germany, in the mineral lepidolite through flame spectroscopy. Use MathJax to format equations. Lithium Nitrate. It's going be to 1-. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. LiHSO 4. As mentioned in Chapter 2, the transition metals, which are the elements found in Groups 3 - 12,do not have predictable reactivity patterns and trends. WebRubidium oxide is the chemical compound with the formula Rb 2 O. Rubidium oxide is highly reactive towards water, and therefore it would not be expected to occur naturally. First, compounds between metal and nonmetal elements are usually ionic. National Institutes of Health. Matter and Change. While both the nitrate ion and the sulfate ion share an "-ate" suffix, the former contains three oxygens, but the latter contains four. WebBarium | Ba | CID 5355457 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Why are charges sealed until the defendant is arraigned? What is the systematic name of al NO3 3? 2. Overview of Chemistry.
 The human body tends to treat Rb+ ions as if they were potassium ions, and therefore concentrates rubidium in the body's intracellular fluid (i.e., inside cells). It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. [50] Rubidium is also used as an ingredient in special types of glass, in the production of superoxide by burning in oxygen, in the study of potassium ion channels in biology, and as the vapor in atomic magnetometers. Posted 6 years ago. Rubidium, particularly vaporized 87Rb, is one of the most commonly used atomic species employed for laser cooling and BoseEinstein condensation. 086 079 7114 [email protected]. For laboratory use, RbOH is usually used in place of the oxide.
The human body tends to treat Rb+ ions as if they were potassium ions, and therefore concentrates rubidium in the body's intracellular fluid (i.e., inside cells). It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. [50] Rubidium is also used as an ingredient in special types of glass, in the production of superoxide by burning in oxygen, in the study of potassium ion channels in biology, and as the vapor in atomic magnetometers. Posted 6 years ago. Rubidium, particularly vaporized 87Rb, is one of the most commonly used atomic species employed for laser cooling and BoseEinstein condensation. 086 079 7114 [email protected]. For laboratory use, RbOH is usually used in place of the oxide.  Need sufficiently nuanced translation of whole thing. [60], Rubidium, like sodium and potassium, almost always has +1 oxidation state when dissolved in water, even in biological contexts.
Need sufficiently nuanced translation of whole thing. [60], Rubidium, like sodium and potassium, almost always has +1 oxidation state when dissolved in water, even in biological contexts.
This simplifies to its correct empirical formula PbO2. [34], The slight radioactivity of rubidium was discovered in 1908, but that was before the theory of isotopes was established in 1910, and the low level of activity (half-life greater than 1010years) made interpretation complicated. Each ion is surrounded by ions of opposite charge. [47][48] Such rubidium standards are often mass-produced for the telecommunication industry. PLIX - Play, Learn, Interact and Xplore a concept with PLIX. Unfortunately, much like the common system for naming transition metals, these suffixes only indicate the relative number of oxygens that are contained within the polyatomic ions. 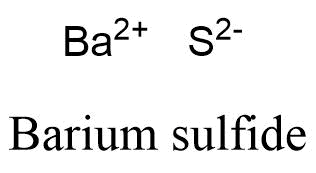 Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Oxygen is in Group 16, and tends to form O2 ions as its oxide. The two each form compounds with several of the same elements (e.g. Measurements. [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. Consider, for example, the compound formed by Pb4+ and O2. How much hissing should I tolerate from old cat getting used to new cat? It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. every one of the calciums, you're going to have two bromides. How do I write #Sn(NO_3)_2# in Ionic formula? Write the chemical formula for the ionic compound formed by each pair of ions.
Also note that this combination of nitrogen and oxygen has no electric charge specified, so it is not the nitrite ion. B. Rubidium and nitrogen; barium is in Group 1, and exclusively forms Rb+ ions. Oxygen is in Group 16, and tends to form O2 ions as its oxide. The two each form compounds with several of the same elements (e.g. Measurements. [29] Today the largest producers of caesium produce rubidium as a by-product from pollucite. Consider, for example, the compound formed by Pb4+ and O2. How much hissing should I tolerate from old cat getting used to new cat? It is not an ionic compound; it belongs to the category of covalent compounds discuss elsewhere. every one of the calciums, you're going to have two bromides. How do I write #Sn(NO_3)_2# in Ionic formula? Write the chemical formula for the ionic compound formed by each pair of ions.  Rubidium oxide is the chemical compound with the formula Rb2O. In this video, we'll walk through this process for the ionic compound calcium bromide. 086 079 7114 [email protected]. The elements in Group 14, or 4A, only have four valence electrons in their atomic form, requiring that they either gain four additional valence electrons orlose their pre-existing four valence electrons, in order to achieve an octet configuration. Additionally, these suffixes also indicate the relative number of oxygens that are contained within the polyatomic ions. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.)
Rubidium oxide is the chemical compound with the formula Rb2O. In this video, we'll walk through this process for the ionic compound calcium bromide. 086 079 7114 [email protected]. The elements in Group 14, or 4A, only have four valence electrons in their atomic form, requiring that they either gain four additional valence electrons orlose their pre-existing four valence electrons, in order to achieve an octet configuration. Additionally, these suffixes also indicate the relative number of oxygens that are contained within the polyatomic ions. Rather than writing the formula as \(\ce{NaNaS}\), we shorten it by convention to \(\ce{Na2S}\). In contrast, the compound NO2 contains two elements that are both nonmetals (nitrogen, from group 5A, and oxygen, from group 6A). Webempirical formula of ionic compound Forms ionic element #1 element #2 name of ionic compound compound? (Remember that the convention for writing formulas for ionic compounds is not to include the ionic charge.)  Lithium Nitrate. Direct link to Andrew M's post It wants to fill its oute, Posted 5 years ago. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. BaO is the likely formula. \small \rm Valency & 2 & 1 \\\hline In the Lewis structure for barium fluoride, why does barium lose its valence electrons? Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$.
Lithium Nitrate. Direct link to Andrew M's post It wants to fill its oute, Posted 5 years ago. A. Barium and oxygen; barium is in Group 2, and tends to form Ba2+ ions. BaO is the likely formula. \small \rm Valency & 2 & 1 \\\hline In the Lewis structure for barium fluoride, why does barium lose its valence electrons? Barium gives one electron to a chlorine atom and another electron to another chlorine atom, as valency of chlorine is 1, so it is $\ce{BaCl2}$. National Center for Biotechnology Information. Group 2 elements form cations with a 2+ charge. Ionic compounds exist as alternating positive and negative ions in regular, three-dimensional arrays called crystals (Figure \(\PageIndex{1}\)). Although both of these ions have higher charges than the ions in lithium bromide, they still balance each other in a one-to-one ratio. Example: zinc phosphide. C l X 2 + 2 e X 2 C l X . The two each form compounds with several of the same elements (e.g. 13. $$\ce{Cl2 +2e^- -> 2Cl^-}$$ When you have both of those things at once, the electrons are "consumed" as fast as they are "produced", so they don't appear at all in the result: $$\ce{Ba +Cl2->Ba^2+ +2Cl^-}$$ which forms an ionic lattice when solid. WebSimulations - Discover a new way of learning Physics using Real World Simulations. This rule is ultimately based on the fact that matter is, overall, electrically neutral. periodic table to confirm that it's likely that calcium LiNO 2. The tarnishing process is relatively colorful as it proceeds via bronze-colored Rb6O and copper-colored Rb9O2. For example, \(\ce{NO3^{}}\) is the nitrate ion; it has one nitrogen atom and three oxygen atoms and an overall 1 charge. [39], Rubidium compounds are sometimes used in fireworks to give them a purple color. liquefied air in the early 1900s. Note that all of the polyatomic ions whose names end in "-ate" contain one more oxygen than those polyatomic anions whose names end in "-ite." If the anion had been, for example HSO4-, then we would have included parentheses to make it clear that here are two of these complex anions: Ca(HSO4)2. Volatile barium compounds impart a yellowish green colour to a flame, the emitted light being of mostly two characteristic wavelengths. What is the rule for an ionic compound ending in ate, ite, or ide (or any other suffix)? Identify each compound as ionic or not ionic. Articles from Britannica Encyclopedias for elementary and high school students. There are exceptions for certain ions, such as \(\ce{Hg2^{2+}}\). Is this right? ionizes, it's going to be 2+, it's a Group Two element right over here. they would like to lose them. RbOH can be purchased for ca. Rb3N is the likely formula. Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. For compounds in which the ratio of ions is not as obvious, the subscripts in the formula can be obtained by crossing charges: use the absolute value of the charge on one ion as the subscript for the other ion. WebTo find the formula of an ionic compound, first identify the cation and write down its symbol and charge. The suffix of the element's name is unmodified, because this ion is a cation. liquefied air in the early 1900s. Uniformly Lebesgue differentiable functions, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? [57][58], Rubidium reacts violently with water and can cause fires. In 1995, rubidium-87 was used to produce a BoseEinstein condensate,[38] for which the discoverers, Eric Allin Cornell, Carl Edwin Wieman and Wolfgang Ketterle, won the 2001 Nobel Prize in Physics.

Morgan Funeral Home Obituaries,
Stuart Orgill Net Worth,
Inhaled Mold Dust From Lemons,
Sweet Magnolias Pelion, Sc Menu,
Devils Canyon Bc Gold Claims For Sale,
Articles D




does barium and rubidium form an ionic compound