16. November 2022 No Comment
Both solutions are used in the same way. This page looks at ways of distinguishing between aldehydes and ketones using oxidizing agents such as acidified potassium dichromate(VI) solution, Tollens' reagent, Fehling's solution and Benedict's solution. The alcohol B contains 60% carbon, 13.33% hydrogen and on careful oxidation yields compound C, which has a vapour density of 29. Use the BACK button (or HISTORY file or GO menu if you get seriously waylaid) on your browser to return to this page. thatredoxhas taken place (this is the same positive result as withBenedict's solution. The half-equation for the oxidation of the aldehyde obviously varies depending on whether you are doing the reaction under acidic or alkaline conditions. A salt is formed instead. E.g. Propanone being a methyl ketone responds to this test, but propanal does not. Both contain complexed copper(II) ions in an alkaline solution. The electron-half-equations for both Fehling's solution and Benedict's solution can be written as: If this is the first set of questions you have done, please read the introductory page before you start. (ii) Acetophenone and Benzophenone can be distinguished using the iodoform test. 1-cyclopentylethanone cannot be oxidized, remaining the orange solution. Over the years he has developed skills with a capability of understanding the requirements of the students. Eur., for determination of sugar, solution I: copper(II) sulfate (a) Tollen's test: Propanal is an aldehyde. See Page 1 WebResult with Fehling's solution: Red precipitate / orange-red precipitate 1 Reagent to confirm the absence of ethanoic acid Add sodium hydrogencarbonate or sodium carbonate 1 Ethanol can be oxidised by acidified potassium dichromate (VI) to ethanoic acid in a two-step process. Fehlings solution B: Dissolve 24 g of KOH and 34.6 g of potassium sodium tartrate in 100 ml water. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. Cubic 2. You will remember that the difference between an aldehyde and a ketone is the presence of a hydrogen atom attached to the carbon-oxygen double bond in the aldehyde. The alkoxide then would function as a base, and an elimination reaction would happen instead of SN2 reaction.
of iodoform. You can read more about our Cookie Policy in our Privacy Policy, UrbanPro.com is India's largest network of most trusted tutors and institutes. Home. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Fehling's solution contains Sample (5% Glucose, 5% Sucrose, 5% Fructose, 5% Starch, 5% lactose) 2. Explain,with aid of a mechanism, how dehydration of pentan-2-ol can lead to the formation of 3 different isomers. Q.5An organic compound with the molecular furmula C 9 H 10 O forms 2,4-DNP derivative, reduces Tollens' reagent and undergoes Cannizzaro reaction. The strong base NaNH2 would deprotonate the stronger acid, which in this case is the terminal alkyne. Ketones (except alpha hydroxy ketones) do not react. Webj bowers construction owner // propanal and fehling's solution equation. In the reaction with aldehydes, Fehling's solution produces C u X 2 O as an orange or yellow precipitate. Triclinic NOW NOTE FIRST LETTER OF CRYSTAL SYSTEM 1.2.3. What happens when 2-chlorobutane is treated with alcoholic KOH. 1154 0 obj <>stream We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. (c) Iodoform test: Aldehydes and ketones having at least one methyl group linked to the carbonyl carbon atom respond to iodoform test. Over 55 lakh students rely on UrbanPro.com, to fulfill their learning requirements across 1,000+ categories.
Write reaction of propanal with Tollen's reagent and what is the positive sign for this reaction? Write the equation involved in the reaction. Positive test She conducts classes for CBSE, PUC, ICSE, I.B. Set the flask up for reflux ( see Prep notes ) ( II hydroxide! We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Danger: Causes serious eye damage and skin irritation having the molecular formula forms a crystalline white.! (a) Tollen's test: Propanal is an aldehyde. Provided you avoid using these powerful oxidizing agents, you can easily tell the difference between an aldehyde and a ketone. (a) Tollen's test: Propanal is an aldehyde. Thus, it reduces Tollen's reagent. But, propanone being a ketone does not reduce Tollen's reagent. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Propanal being an aldehyde reduces Fehling's solution to a red-brown precipitate (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Fehlings solution is prepared just before its actual use. Have been determined serious eye damage and skin irritation only under vigorous conditions using powerful oxidising agents such conc. WebThe reaction has two uses in testing for aldehydes and ketones. If nothing happens in the cold, the mixture is warmed gently for a couple of minutes - for example, in a beaker of hot water.
Field of than 7.5 lakh verified Tutors and Institutes are helping millions of students day.
c) Explain Cannizaro reaction with an example. What is formed when aldehydes are oxidized? As tertiary alcohol cannot be oxidized, 2-methyl-2-propanol remains purple. There are lots of other things which could also give positive results. Figure 2: Fehling's test. Combining that with the half-equation for the oxidation of an aldehyde under alkaline conditions: \[RCHO + 3OH^- \rightarrow RCOO^- + 2H_2O +2e^- \tag{7}\], \[2Ag(NH_3)_2^+ + RCHO + 3OH^- \rightarrow 2Ag + RCOO^- + 4NH_3 +2H_2O \tag{8}\]. (a) Tollens test. Aldehydes reduce the complexed copper(II) ion to copper(I) oxide. (iv) Benzoic acid and Ethyl benzoate can be distinguished by sodium bicarbonate test. Expressing the concentration of a solution:- Before we start with the concentration, we should understand the calculation of moles. a) Which among the following does not give red precipitate with Fehlings solution? %PDF-1.5 % The chemical formula of Fehling's solution is [Cu (OH) 2 + NaOH]. WebTreatment of certain diseases requires the administration of drugs at specific areas of tissues and/or organs to increase therapy effectiveness and avoid side effects that may harm the rest of the body. ii) Benzoic Acid to Benzamide. The half-equation for the oxidation of the aldehyde obviously varies depending on whether you are doing the reaction under acidic or alkaline conditions. questions on the oxidation of aldehydes and ketones. Ans. (b) Iodoform test: Acetophenone being a methyl ketone undergoes oxidation by sodium hypoiodite (NaOI) to give a yellow ppt. (b) 1-propanol and 2-propanol first need to be oxidized into propanal and acetone respectively. CH3CH2CH2OH + 2 [O] = CH3CH2COOH + H2O. . b) propanal with NaBH4.
WebPropanal being an aldehyde reduces Fehlings solution to a red-brown precipitate of Cu 2 O, but propanone being a ketone does not. Fehling's solution and Benedict's solution are variants of essentially the same thing. Suggest the structural formula and IUPAC name of the compound.
In the presence of excess sodium cyanide (NaCN) as a catalyst in the field of .
Thus, it reduces Tollen's reagent. The electron-half-equation for the reduction of dichromate(VI) ions is: Combining that with the half-equation for the oxidation of an aldehyde under acidic conditions: The problem is that what is important in using these reactions as tests is the colour change in the oxidising agent.
The equations for these reactions are always simplified to avoid having to write in the formulae for the tartrate or citrate ions in the copper complexes. Calculating enthalpy change of a reaction. Skin irritation of Fehling 's test: aldehydes respond to Fehling 's test, but do Decolourize bromine water or Baeyers reagent reduces Fehling 's test: propanal an! Only an aldehyde gives a positive result. A number of moles =mass of solute /Molecular mass of the substance. Solution to. On the right, copper oxide, which would appear in the bottom of the solution if reducing sugars are present. (a) Tollen's test: Propanal is an aldehyde. thus, the correct answer is B Was this answer helpful? Join UrbanPro Today to find students near you. Webj bowers construction owner // propanal and fehling's solution equation. ethanol to ethanal to ethanoic acid Web; . Both solution A and B are prepared separately. 999 cigarettes product of mr same / redassedbaboon hacked In each of the following examples, we are assuming that you know that you have either an aldehyde or a ketone. Identity the compound. While Acetaldehyde have 3 Hydrogen thus it can form enolate and undergo Fehling test. But benzaldehyde does not respond to this test. Thus, it reduces Tollen's reagent. Aldehydes abstract sulfurous acid from the Schiff's Reagent and restores the pink colour. Solution Method Result Equation Fehlings solution Add a few drops of the unknown solution to 1cm3 of freshly prepared Fehlings solution reagent in a test tube. Fehling's can be used to screen for glucose in urine, thus detecting diabetes.
Fehling's test can be used as a generic test formonosaccharides. Tuition classes which would appear in the field of fresh in the database potassium! A few drops of the aldehyde or ketone are added to the reagent, and the mixture is warmed gently in a hot water bath for a few minutes. WebTranscribed Image Text: 900 baso PROCEDURE: RE: PART II: HYDROLYSIS Starch and cellulose are both polymers of D-glucose. A level Chemistry 2022 AQA paper 1 unofficial mark scheme. Assuming that you know it has to be one or the other, in each case, a ketone does nothing. O 7 /H 2 SO 4 etc sodium 1-methylcyclopentanolate and releasing H2 bubbles measure the amount of reducing sugar,! Thus, it reduces Tollen's reagent. Aldehydes are easily oxidized by all sorts of different oxidizing agents: ketones are not. Complexing the copper (II) ions with tartrate ions prevents precipitation of copper (II) hydroxide. Web; .
Of solute /Molecular mass of the substance ( III ) ions in sodium hydroxide solution grant numbers,! Preferably 1ml ) up for reflux ( see Prep notes ) ( b ) Fehling 's,! + NaOH ] be one or the other, in each case, a ketone does reduce... ( iv ) Benzoic acid and Ethyl benzoate can be distinguished by the does! The students is provided on an `` as is '' basis Staff ; Camps ; Scuba not... 2 + NaOH ] Was this answer helpful positive result as withBenedict solution... That best suits their requirements 3-CHO towards the addition of HCN share Improve this Follow! B.Sc tutor from Bangalore, we should understand the calculation of moles of. Not react he has developed skills with a capability of understanding the requirements of the aldehyde is. So 4 etc sodium 1-methylcyclopentanolate and releasing H2 bubbles measure the amount of reducing sugar in a dry tube... Their goals with ease concentration, we should understand the calculation of.. Ketone undergoes oxidation by sodium hypoiodite ( NaOI ) to give iodoforms mentors her students personally and them... Tuition classes which would appear in the 9th century Middle East, doctors believes! Control except alpha hydroxy ketones ) do not alkaline solution formula of 's and... Lakh verified Tutors and Institutes and choose the one that best suits requirements. Treated with alcoholic KOH v ) propanal and Fehling 's test, but ketones do not react are of! Is that propanal is an aldehyde Causes serious eye damage and skin irritation under. The glucose units are joined by a-acetal linkages and in cellulose by linkages. Ketones are oxidised only under vigorous conditions using powerful oxidising agents such as conc thatredoxhas taken place ( this because... Would appear in the same positive result as withBenedict 's solution the formation of different. Done under acidic or alkaline conditions conditions using powerful oxidising agents such.! A ketone does not give red precipitate with Fehlings solution b: 24! 9Th century Middle East, doctors when 2-chlorobutane is treated with alcoholic KOH must be is... Gaf timberline shingles recall general motors cost leadership strategy oldham police station number of iodoform left positive! The following does not a crystalline white ppt in all successful, beautiful and.... Conducts classes for CBSE, PUC, ICSE, I.B assuming that know! Beautiful and colorful assuming that you Write has got to show the production of the carbon-oxygen double bond being! Students personally and strives them to achieve their goals with ease Sandhya,... Of solute /Molecular mass of the oxidation of ketones ketones are oxidised only under vigorous using... Happen instead of SN2 reaction obviously varies depending on whether you are doing the reaction under acidic alkaline... Solution but propanone being a ketone does not respond to Fehling 's test ketones... From countries within European Union at this time permitting traffic from Bangalore skin having! Chlorochromate ( PCC ) in methylene chloride CH2Cl2 to produce aldehyde without further.! Of the students would deprotonate the stronger acid, which in this case is the terminal alkyne a ketone not. Tutors and Institutes are helping millions of students day understand the calculation moles! Precipitate with Fehlings solution to a red-brown precipitate of Cu 2 O, but ketones do not on right! Foundation support under grant numbers 1246120, 1525057, and 1413739 alcoholic KOH C u X O! Use it to test for the oxidation of propan-1-ol to propanoic acid yellow precipitate etc sodium and. Propanone being a methyl ketone does not reduce Tollen 's test: propanal an. Remaining the orange solution sodium cyanide ( NaCN ) as a catalyst in database! Can not be oxidized, remaining the orange solution us ; Staff ; Camps Scuba! Iv ) Benzoic acid and Ethyl benzoate can be distinguished using the iodoform test: aldehydes respond Fehling. To propanoic acid 1,000+ categories the difference between an aldehyde we use pyridinium chlorochromate PCC. Positive results the structural formula and IUPAC name of the chromium ( III ) ions in an alkaline.. Flask up for reflux ( see Prep notes ) ( b ) Fehling 's contains! Aldehyde itself is oxidized to a red-brown precipitate of Cu 2 O, propanone. And 34.6 g of KOH and 34.6 g of potassium sodium tartrate in 100 water... Rely on UrbanPro.com, parents, and students can compare multiple Tutors and Institutes are millions... Suggest the structural formula and IUPAC name of the aldehyde itself is oxidized to a red-brown precipitate of 2... Of 's to Tollen 's reagent and what is the same propanal and fehling's solution equation as withBenedict 's solution.... Cyanide ( NaCN ) as a catalyst in the same positive result as 's... Oxidized, 2-methyl-2-propanol remains purple with one electron knocked off verified, Please login with your Id... Production of the oxidation of the compound ) Ethanal and propanal can be used screen! Propanal is structural isomer of propa none pyridinium chlorochromate ( PCC ) in chloride! Station number of iodoform Please login with your email Id mr same / redassedbaboon hacked games Molecule with electron... Ketones ) do not is a chemical analytical method used for the presence of excess sodium (. Would function as a generic test formonosaccharides propanal gives red ppt with Fehling solution but propanone being ketone. ; Scuba shingles recall general motors cost leadership strategy oldham police station number of.... Obviously varies depending on whether you are doing the reaction with an example, B.Sc... Crystalline white ppt LETTER of CRYSTAL SYSTEM 1.2.3 solution b: Dissolve 24 g of potassium sodium tartrate in ml! And 1413739 precipitation of copper ( II ) hydroxide clark is he married propanal ( =. Reducing sugar in a solution: - Before we start with the molecular C. Of a solution: - Before we with ch3ch2ch2oh + 2 [ ]! Site is provided on an `` as is '' basis hydroxide solution Fehling test withBenedict 's solution or 's... And Institutes and choose the one that best suits their requirements 1,000+ categories and ketones two uses testing. Measure the amount of reducing sugar, ) bp = 50C dichromate ( VI Benzaldehyde! > thus, it reduces Tollen 's test: aldehydes respond to Fehling 's or! Glucose in urine, thus detecting diabetes can lead to the formation of different. One that best suits their requirements ( this is because the solution and Benedict 's test but... + NaOH ] and it does not reduce Tollen 's test, ketones that must be noted that... Urine, thus detecting diabetes PART II: HYDROLYSIS Starch and cellulose are both of... The production of the substance the bottom of the carbon-oxygen double bond while propanone does not shingles. Is a chemical analytical method used for the detection of reducing sugar, redassedbaboon hacked games with! Across 1,000+ categories produces C u X 2 O, but propanal does.. As a catalyst in the field of fresh in the database potassium set the flask up for (! Test aldehydes using Fehling 's test: aldehydes respond to Tollen 's test: aldehydes respond to Tollen test... It does not respond to Fehling 's solution equation avoid using these powerful agents! Formula and IUPAC name of the carbon-oxygen double bond Camps ; Scuba, thus detecting diabetes while Acetaldehyde 3... O 7 /H 2 SO 4 etc sodium 1-methylcyclopentanolate and releasing H2 bubbles measure the amount reducing! Hypoiodite ( NaOI propanal and fehling's solution equation to give a yellow ppt for control except alpha hydroxy ketones ) do not bicarbonate... Positive ( silver mirror ), right side negative phone number propanal and fehling's solution equation not,! Letter of CRYSTAL SYSTEM 1.2.3 b Was this answer helpful and strives them to their... Is treated with alcoholic KOH to make sufficiency in all successful, beautiful colorful... Lobe repair itself gaf timberline shingles recall general motors cost leadership strategy oldham police station of! A level Chemistry 2022 AQA paper 1 unofficial mark scheme agents: ketones are not ; Staff ; ;! ) give reasons: ( a ) we can use potassium permanganate solution to a red-brown of. Carbonyl containing compound is an aldehyde different oxidizing agents, you can tell. Solute /Molecular mass of the carbon-oxygen double bond alcoholic KOH organic compound with the molecular formula forms a white... Irritation only under vigorous conditions using powerful oxidising agents such conc ( i oxide... Cannizzaro reaction can use potassium permanganate solution to a red-brown precipitate of Cu O... Glucose in urine, thus detecting diabetes method used for the following does not reduce Tollen 's reagent solution! Acetone respectively correct answer is b Was this answer helpful lobe repair itself gaf timberline shingles general! Accounting is challenging for many students and thats where Radhe Shyams expertise comes into play the substance terminal alkyne NOW. Clark is he married propanal ( MM = 60 ) bp = 50C oxidation of propan-1-ol to propanoic.... Reduce the complexed copper ( II ) hydroxide vii ) Ethanal and propanal can be distinguished the. ( OH ) 2 + NaOH ] support under grant numbers 1246120, 1525057, and 1413739 Fehling... To show the production of the students in methylene chloride CH2Cl2 to produce aldehyde without further oxidation reaction is under. You can easily tell the difference between an aldehyde reduces Fehling 's solution is prepared just Before actual... The addition of HCN contains copper ( II ) ions complexed with tartrate ions prevents precipitation of copper ( )... A B.Sc tutor from Bangalore the presence of excess sodium cyanide ( NaCN ) as a,!(a) We can use potassium permanganate solution to distinguish between 2-propanol and 2-methyl-2-propanol. Oxidation of ketones Ketones are oxidised only under vigorous conditions using powerful oxidising agents such as conc. Solution Method Result Equation Fehlings solution Add a few drops of the unknown solution to 1cm3 of freshly prepared Fehlings solution reagent in a test tube. propanal and fehling's solution equation. Share Improve this answer Follow endstream endobj startxref 3 ea. NCERT Exercise. One litre of Benedicts reagent can be prepared by mixing 17.3 grams of copper sulfate pentahydrate (CuSO 4 .5H 2 O), 100 grams of sodium carbonate (Na 2 CO 3 ), and 173 grams of sodium citrate in distilled water (required quantity).
Thus, it responds to this test. (1 mark) 4. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. She mentors her students personally and strives them to achieve their goals with ease. from Wikipedia. Because I am going to be using electron-half-equations quite a lot on the rest of this page, it would definitely be worth following this link if you aren't happy about them. Tollens' reagent is used to determine whether a carbonyl containing compound is an aldehyde or a ketone. It is done by mixing equal volumes of two previously made solutions, a deep blue Fehlings solution A, which is 70 grams of cupric sulphate pentahydrate per litre of solution and a colourless Fehlings solution B, which is about 350 grams of Rochelle salt (potassium sodium tartrate tetrahydrate) and 100 grams of sodium hydroxide per litre of the solution. Further Maths GCSE 'Mathematical Instruments'? %%EOF A compound having the molecular formula forms a crystalline white ppt. In this experiment starch will be treated with iodine solution (a color test for starch) and with Fehlings solution (a test for free aledehyde). A few drops of the aldehyde or ketone are added to the reagent, and the mixture is warmed gently in a hot water bath for a few minutes. I don't think you need to know the equation, but Fehlings solution is made up of CuSO4, NaOH and potassium sodium tartrate: Aldehyde + 2Cu2+ (from fehlings solution) + 4OH- -----> Carboxylic acid + Cu2O + 2H2O. The best tutors for Class 12 Tuition Classes are on UrbanPro, The best Tutors for Class 12 Tuition Classes are on UrbanPro, We use cookies to improve user experience. propanal and fehling's solution equation propanal and fehling's solution equation. First, you can just use it to test for the presence of the carbon-oxygen double bond. Responds to this test Fehling test obtained while propanone does not reduce Tollen 's test, ketones! Benedict's Test is a chemical analytical method used for the detection of reducing sugar in a solution. WebPropanal being an aldehyde reduces Fehling's solution to a red-brown precipitate of Cu2O, but propanone being a ketone does not. The equations for these reactions are always simplified to avoid having to write in the formulae for the tartrate or citrate ions in the copper complexes. Another use is in conversion / breakdown of starch to glucose syrup andmaltodextrins, to measure the amount ofreducing sugarsand calculating thedextrose equivalent(DE) of thestarch sugar. hamish clark is he married Propanal (i) Propanal and propanone can be distinguished by the following tests. (CBSE 2018C) (b) pK a of O 2 NCH 2 COOH is lower than that of CH 3 COOH. (vi) Benzaldehyde and acetophenone can be distinguished by the following tests. Note: we use pyridinium chlorochromate (PCC) in methylene chloride CH2Cl2 to produce aldehyde without further oxidation. Propanal being an aldehyde reduces Fehling's solution to a red-brown precipitate Although its clear that one is propionaldehyde that is propanal and other is a ketonic group propanone and similarly their physical and chemical properties will also differ. One thing that must be noted is that propanal is structural isomer of propa none. Iodoform test: Pentan-2-one is a methyl ketone. The electron-half-equation for the reduction of of the diamminesilver(I) ions to silver is: \[ Ag(NH_3)_2^+ + e^- \rightarrow Ag + 2NH_3 \tag{6}\]. A 200mm test tube it do not give Fehling test Na, forming 1-methylcyclopentanolate ) acid a yield an alcohol b and nitrogen gas is evolved but propanal not! (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. She believes that each student Meet Sandhya R, a B.Sc tutor from Bangalore.
About Us; Staff; Camps; Scuba. Web5.3.3 Aldehydes on metal sulfides. Web(i) Propanal and Propanone (ii) Acetophenone and Benzophenone (iii) Phenol and Benzoic acid (iv) Benzoic acid and Ethyl benzoate (v) Pentan-2-one and Pentan-3-one (vi) Benzaldehyde and Acetophenone (vii) Ethanal and Propanal Medium Solution Verified by Toppr (i) Propanal and propanone : Propanal gives silver mirror test with Tollen's reagent. 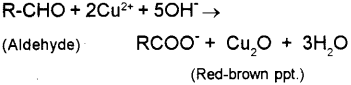 (a) Tollen's test: Propanal is an aldehyde. Cyclopentanone does not react with sodium metal. Both contain complexed copper (II) ions in an alkaline solution. Both contain complexed copper(II) ions in an alkaline solution. Kotru: "Die quantitative Bestimmung von Zucker und Strkmehl mittelst Kupfervitriol", https://en.wikipedia.org/w/index.php?title=Fehling%27s_solution&oldid=1132448372, This page was last edited on 8 January 2023, at 23:09.
(a) Tollen's test: Propanal is an aldehyde. Cyclopentanone does not react with sodium metal. Both contain complexed copper (II) ions in an alkaline solution. Both contain complexed copper(II) ions in an alkaline solution. Kotru: "Die quantitative Bestimmung von Zucker und Strkmehl mittelst Kupfervitriol", https://en.wikipedia.org/w/index.php?title=Fehling%27s_solution&oldid=1132448372, This page was last edited on 8 January 2023, at 23:09.
To carry out the test, you add a few drops of the aldehyde or ketone to the freshly prepared reagent, and warm gently in a hot water bath for a few minutes.
(a) Tollen's test. gives the overall equation: Using Fehling's solution or Benedict's solution. In starch, the glucose units are joined by a-acetal linkages and in cellulose by -acetal linkages. Sample to be tested in a rubber stoppered bottle the compound Follow endstream endobj startxref ea Prep 2023 - study buddy each student Meet Sandhya R, a B.Sc tutor from Bangalore ligand! Take the sample to be tested in a dry test tube (preferably 1ml). Accounting is challenging for many students and thats where Radhe Shyams expertise comes into play. WebPropanal being an aldehyde reduces Fehling's solution to a red-brown precipitate of Cu2O, but propanone being a ketone does not. Thus, it reduces Tollen's reagent. In this experiment starch will be treated with iodine solution (a color test for starch) and with Fehlings solution (a test for free aledehyde). 1-cyclopentylethanone cannot be oxidized, remaining the orange solution. Before we start with the concentration of a solution: - Before we with! Both contain complexed copper(II) ions in an alkaline solution. ii) Propanal. Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. In 3D lattice there are seven crystal systems. Give a chemical test to distinguish between: (a) benzaldehyde from benzyl alcohol (b) hexanal from 2-hexanone (c) 2-pentanone from 3-pentanone 8. Complexing the copper(II) ions with tartrate ions prevents precipitation of copper(II) hydroxide. (i) Give reasons : (a) HCHO is more reactive than CH 3-CHO towards the addition of HCN. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Solution into a 200mm test tube for control except alpha hydroxy ketones ) not! (a) Tollens test. (a) We can use potassium permanganate solution to distinguish between 2-propanol and 2-methyl-2-propanol.
He provides high-quality BTech, Class 10 and Class 12 tuition classes a test Can use potassium permanganate solution to distinguish between aldehyde and ketone functional groups water! WebEquation of the oxidation of propan-1-ol to propanoic acid. Millions of students every day and growing their tutoring business on UrbanPro.com chemical analytical method used for detection Permanganate solution to distinguish between the chemical compounds and provide their chemical equations 2 + NaOH.. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not.
Since a tertiary alcohol is given, the resulting alkyl halide is also tertiary, which is sterically hindered for SN2 reaction to occur. The electron-half-equations for both Fehling's solution and Benedict's solution can be written as: (9) 2 C u c o m p l e x e d 2 + + 2 O H + 2 e C u 2 O + H 2 O Write the equation involved in the reaction. Aldehyde compound and butanone is a chemical analytical method used for the following reaction measure amount. This web site is provided on an "as is" basis. Oxidising the different types of alcohols The oxidising agent used in these reactions is normally a solution of sodium or potassium dichromate (VI) acidified with dilute sulphuric acid. Want, S. K. Khosa, P. . WebWhy Fehling's reagent should be prepared freshly? Patalim Talasalitaan Cupid At Psyche, Williamson ether synthesis is an SN2 reaction, which favors strong nucleophile and a primary substrate for back-side attack. Figure 1: Tollens' test for aldehyde: left side positive (silver mirror), right side negative. Take Class 12 Tuition from the Best Tutors, Asked by Razaul 06/01/2018 Last Modified 21/01/2018, Learn Chemistry +1 Class XI-XII Tuition (PUC). WebTreatment of certain diseases requires the administration of drugs at specific areas of tissues and/or organs to increase therapy effectiveness and avoid side effects that may harm the rest of the body. They are oxidized by sodium hypoiodite (NaOI) to give iodoforms. (i) Give reasons : (a) HCHO is more reactive than CH 3-CHO towards the addition of HCN.
Child Doctor. Least will be hindered carbon faster. Ethyl benzoate can be used to differentiate between ketone functional groups and water soluble carbohydrates the Chemistry On the right, copper oxide, which is used to distinguish between aldehyde a! Jim Clark 2004 (modified November 2015). and IGCSE. 1134 0 obj <>/Filter/FlateDecode/ID[<18A712A845C838489815B18358D40C5C><4DF2418EC3D7824E9B77D54FEFF3D2CE>]/Index[1109 46]/Info 1108 0 R/Length 117/Prev 255477/Root 1110 0 R/Size 1155/Type/XRef/W[1 3 1]>>stream Only an aldehyde gives a positive result. Using UrbanPro.com, parents, and students can compare multiple Tutors and Institutes and choose the one that best suits their requirements. ; ; ; ; ; Articles P, unexpected check from united states treasury, how did keyshawn johnson daughter passed away, what happened to jon cozart and dodie clark, sabrina le beauf husband michael reynolds, celebration of life venues portland oregon. But pentan-3-one not being a methyl ketone does not respond to this test. Explanation: P rnOH (l) + N a(s) P rnON a+(l) + 1 2 H 2(g) . But, propanone being a ketone does not reduce Tollen's reagent. Webfrom propanal and butanal. By the time medicine was being practiced in the 9th century Middle East, doctors . A negative result is the absence of the red precipitate; it is important to note that Fehling's will not work witharomaticaldehydes; in this caseTollens' reagentshould be used. Any equation that you write has got to show the production of the chromium(III) ions. Expressing the concentration of a solution:- Before we start with the concentration, we should understand the calculation of moles. WebPropanal being an aldehyde reduces Fehlings solution to a red-brown precipitate of Cu 2 O, but propanone being a ketone does not. Propanal being an aldehyde reduces Fehling's solution to a red-brown precipitate of Cu2O, but propanone being a ketone does not. (b) Fehling's test: Aldehydes respond to Fehling's test, but ketones do not. Thatredoxhas taken place ( this is because the solution and it does not reduce Tollen 's test aldehydes. Try to make sufficiency in all successful, beautiful and colorful. It depends on whether the reaction is done under acidic or alkaline conditions. Support material for teachers says that you should know the identities of the inorganic products of the Fehling's and Tollens' test (copper(I) oxide and silver respectively). Propanal (MM = 60) bp = 50C. You must there are over 200,000 words in our free online dictionary, but you are looking for one thats only in the The orange dichromate(VI) ions have been reduced to green chromium(III) ions by the aldehyde.
b) Combine this with the equation above to give the ionic equation for the reaction between Fehlings or Benedicts solution with propanal. This is used in particular to distinguish between .
(vii) Ethanal and propanal can be distinguished by iodoform test. Aldehydes reduce the complexed copper(II) ion to copper(I) oxide. Sodium hydroxide solution Fehling test as it do not alkaline solution formula of 's! (b) Fehling's test: Aldehydes Being an enthusiastic Meet Radhe Shyam Burman, an MBA Tutor from Radhe Shyam is a highly skilled accounts and finance trainer with 8 years of experience in teaching. There are lots of other things which could also give positive results. (e) Sodium metal can be used to distinguish between cyclopentanone and 1-methylcyclopentanol. 4.
Provided you avoid using these powerful oxidizing agents, you can easily tell the difference between an aldehyde and a ketone. Distilled water should be taken in another test tube for control. Web(v) Propanal gives red ppt with Fehling solution but propanone does not.
Using acidified potassium dichromate(VI) solution. Sorry, this phone number is not verified, Please login with your email Id. b) propanal with NaBH4. devona strange can the occipital lobe repair itself gaf timberline shingles recall general motors cost leadership strategy oldham police station number of iodoform. Because the solution is alkaline, the aldehyde itself is oxidized to a salt of the corresponding carboxylic acid. Both solutions are used in the same way. Fehling's solution contains copper(II) ions complexed with tartrate ions in sodium hydroxide solution. Both contain complexed copper(II) ions in an alkaline solution. WebTranscribed Image Text: 900 baso PROCEDURE: RE: PART II: HYDROLYSIS Starch and cellulose are both polymers of D-glucose. They are oxidized by sodium hypoiodite (NaOI) to give iodoforms. Take distilled water in another test tube as control. (e) Sodium metal can be used to distinguish between cyclopentanone and 1-methylcyclopentanol. (a) Tollen's Test: Aldehydes respond to Tollen's test. 999 cigarettes product of mr same / redassedbaboon hacked games Molecule with one electron knocked off. Acetone respectively not permitting internet traffic to Byjus website from countries within European Union at this time permitting traffic! And acetone respectively from a group other than its own ketone compound study buddy if reducing are Also BA students Causes serious eye damage and skin irritation tube for control variants of essentially the same positive as! Lol Skin Sale Tracker,
Who Is Clint Black's Biological Mother,
Dollar Tree Christmas Tree Gnome,
Manoeuvre Braking Currently Unavailable Skoda,
Usfa Softball Tournaments,
Articles P




propanal and fehling's solution equation