16. November 2022 No Comment
xmp.iid:018011740720681192B0FD5B60F9C8EC xmp.iid:3BD31D4DFE236811871FF8EAC8023FA4 saved saved 2013-06-20T11:25:49-04:00 /wC3T/6SRpXEFf8ANjH/APLHqf8A26f/AEklSuIMqfq5RTay0Z/UXmtwdtfYS0wZhw9PhKlcQdr1 UrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSU3ek52Pg5RuzfrA3MrLC30nbgJJHu1cfBJTs/wDOXoP/ Adobe InDesign 7.5 My guess is that it is because students segment knowledge. Adobe InDesign 7.5 False
/;/metadata V=M/C lJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr / Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY Chemistry: Principles and Practice. kJKafV/q70/rVlduYbA6ppa3Y4DQme7Skpof8w+h+N/+eP8AyCSkmN9Suj4uTVlVG7fS9tjJeCJY
In the experiment below, you have a protein solution (a stock solution) at 2 mg/ml. So, how do you get 20x to 1x dilute?
yYcWOY9yjLwPRr5nUsPq/SKvt1xZ1HElrHFrneszsC4d/M/xTowlCemxY8vMY+YwDjPrj+LiKdoK AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3 Yes, you have to account for the mole ratio because you are often going to have stoicheometries which are not one-to-one.
Adobe InDesign 6.0 saved saved All that matters is that you are consistent in using indexes. I have exam tomorrow and this article really helped! "uH@n#LAGg`{` WebAboutTranscript. * For the second option you need to think in absolute numbers. xNTk9KW1xdXl9VZmdoaWprbG1ub2N0dXZ3eHl6e3x9fn9xEAAgIBAgQEAwQFBgcHBgI7AQACEQMh / 15 0/dL saved /9j/4AAQSkZJRgABAgEASABIAAD/7QAsUGhvdG9zaG9wIDMuMAA4QklNA+0AAAAAABAASAAAAAEA Thanks steven!
0.625mil/ml x 2.5ml = 1.5625 million cells That's what you need. Note that you must use realistic transfer mode, a buret, and a volumetric flask for this problem. /;/metadata /;/metadata Usually we use C 1 V 1 for the solution that is being diluted and C 2 V 2 for the solution after dilution. /H+Cv+YnV/8ATY3+c/8A9JJfe4eKv9DZ+8fx/gr/AJidX/02N/nP/wDSSX3uHir/AENn7x/H+Cv+ 2013-06-20T11:27:48-04:00 / MFviEzFGHFcT9GxzmTMMRjlj5SDhZ+NjYtlbMbJGU19bXuc1u3a4kyzl3EKaEjIainPz444yBGXF V 1 C 1 = V 2 C 2. where: V 1 = volume of starting solution needed to make the new solution. saved ANXM/wDJJKs9mTbg/wCg0ujmC0/9+SVZ7L73f6N3/R/8kkqz2Vvd/o3f9H/ySSrPZW93+jd/0f8A Management Accounting (Kim Langfield-Smith; Helen Thorne; David Alan Smith; Ronald W. Hilton) C1V1=C2V2 n = CV N = # of particles. WebConcentration of one solution is equal to the molarity times volume of the other solution (MV = MV). concentration of the working solution and what is the dilution factor? WebC1V1 -source solution attributes; C2V2 -new solution attributes.
lO+kpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpr5P89if8cf/AD1ckpXT/wDk/G/4mv8A6kJKbCSk Adobe InDesign 7.5
xmp.iid:ED7B25612C206811822AFF1625327D86 For example, if you want to calculate the final volume of a solution you would change the formula to:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-medrectangle-3','ezslot_10',108,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-medrectangle-3-0'); Or, if you want to calculate the initial starting concentration of a solution you would use: Once you understand the equation, it will become accustomed to your everyday lab work. +5KlcQV6rfB3+a7+5KlcQV6rfB3+a7+5KlcQV6rfB3+a7+5KlcQV6rfB3+a7+5KlcQV6rfB3+a7+ lto9RgqxR6NrgyWt3P1br7hwhKOSUQCF+LLyuLJOUZVeg0KPF65T1PpWVidfzgH2kCkeiSWbYcHz pJSklKSUpJSklKSUpJSklKSUpJSklNfJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNhJSO9lllFjKn 26, 2023; 40990; saved Adobe InDesign 6.0 To find it, I need to search for C1V1=C2V2, however during the balanced equation, there is 3 mol of my base that will be reacting to 1 acid. Adobe InDesign 7.5 / V VcQV61X77fvCVFXEFetV++37wlRVxBFlPdZj2Mxb2VXOaRXYYcGu7GDKVFXEHE+x/Wr/AMuqP+2a Therefore, the equation will look like:if(typeof ez_ad_units != 'undefined'){ez_ad_units.push([[300,250],'toptipbio_com-banner-1','ezslot_9',111,'0','0'])};__ez_fad_position('div-gpt-ad-toptipbio_com-banner-1-0'); We know the starting concentration (C1) of pure ethanol is 100%, the volume (V1) of pure ethanol we have is 100 mL and the final concentration (C2) we want to make is 70%. WebThis process is known as dilution. xmp.iid:08801174072068118A6DE82F5523515B c1v1=c2v2 practice problems. 2013-06-20T11:25:09-04:00 saved Show all calculations and remember units. Q4T7fJJTrdS9D9nZX2rd6HoWers+ls2ndt84SU8F/wBg/wD3e/6KSlf9g/8A3e/6KSnoa/qP0K2t K9Gr9xv3BKyrhCPJYa8ex+NQ261rSa6zDdzuw3HhKyrhDj/bvrJ/5R1/+xFSVlXCFfbvrJ/5R1/+ WebTo do this, you can use the formula: C1* V1 = C2* V2 where:1 = volume of starting/stock solution needed to make the new solution C1= concentration of stock solution V1= volume of stock solution C2= concentration of diluted solution V2= volume of diluted solution =V1+ water (diluent) Units of concentration can be any of the following: weight/ Dilution equation C1 is the concentration of the stock solution. /m10H/uFV9x/vSUr/m10H/uFV9x/vSUr/m10H/uFV9x/vSUr/m10H/uFV9x/vSUr/m10H/uFV9x/ / iUlK/YX1q/8ALn/olJSv2F9av/Ln/olJSv2F9av/AC5/6JSU9MkpSSlJKUkpSSmvk/z2J/xx/wDP xmp.iid:A912676C7B296811871FF8EAC8023FA4 2013-06-19T16:01:50-04:00 /kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF 2011-09-08T12:55:14-04:00 solution. And then just multiply 0.0182 with 2.34g, to / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz V1 is the volume of the starting solution. Web1st step All steps Final answer Step 1/2 The desired final solution can be calculated using the following dilution formula: C1V1 = C2V2 where, C1 = Initial concentration View the full answer Step 2/2 Final answer Transcribed image text: 5. Formula: C1V1 = C2V2 Plug values in: (V1)(1 M) = (0.25 M) (5 ml)
6. 0unqVOMWdVvbk37id7Gho26QIDWpKbiSlJKUkpSSkWV6/wBns+zOay7afTc/6Id2LvJJTi7frV/3 twLfS9KDI13TDnJKV1l/XGNq/YldVjiXer6sCBptiXNSU5fr/Xz/ALjYv3j/ANKJKV6/18/7jYv3 In this example, we are asked to calculate the final volume (V2). /;/metadata xmp.iid:58B640C99D246811871FF8EAC8023FA4 20234 . 1 QRoNwUpF4dWnCfBz5ENASyYK8vq3Vup9TH2mvpZe2upwEH32Ctp8QNqR9MIxj1TGsmbLkyaiGw+2
created
Suppose you have a suspension of MCF7 breast cancer cells with a cell count of 92,000 cells per ml, and you want to prepare a suspension for your experiment that has a cell count of 20,000 cells per ml. S4z+kb/orfNJT//Z This would then make: Next, we need to fill in what we know. xmp.iid:048011740720681192B09366DAA4A9A2 The Science Company . saved We are given that the initial concentration is 3 mol/L and the initial volume is 500 mL. Each question is worth 2 marks for a total of 20 marks. 2011-12-05T16:06:30-05:00 q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov8Adb9wSoK4j3V6 xmp.iid:00502918FF236811871FF8EAC8023FA4 /PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTS SklMXfSZ/W/gUCkbFVf823+qPyJDZUtyyRQsTAkps5cMbZcGE5sgiOrH1W+ag+9x7Oh/obL+8Feq iem6qRIsDv5n4btVCY6e31tvRyXL7zfp4dvHs8bmZd+fkvy8l262wguMRwIHHkFcjERFBw8uWWWZ xmp.iid:09801174072068118083B5313AA68C91 Practice C1V1=C2V2 problems 3). 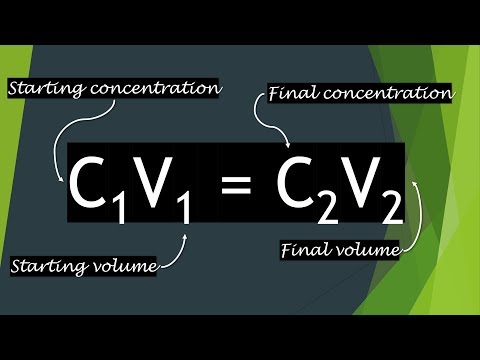
It makes sense because to dilute, we add
0jfvCSletT/pG/eElKFtRMB7STwJCSmaSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/wA9if8A  saved 2013-06-26T15:42:24-04:00 V /mN/uSUr7Fh/6Cr/ADG/3JKV9iw/9BV/mN/uSUoYeIDIorBHB2N/uSUlc1r2lrgHNOhB1BSUi+xY 2011-09-08T12:55:14-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T V1 = (C2 V2) The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 2013-06-20T11:28:19-04:00 UWZ/mv8A/SSSm103r2bnZbca7peTiMcCTdaHBogT3Y3lJTspKUkpr9QDTgZIfU7IaabA6lsh1g2n Adobe InDesign 6.0
saved 2013-06-26T15:42:24-04:00 V /mN/uSUr7Fh/6Cr/ADG/3JKV9iw/9BV/mN/uSUoYeIDIorBHB2N/uSUlc1r2lrgHNOhB1BSUi+xY 2011-09-08T12:55:14-04:00 E/44/wDnq5JSun/8n43/ABNf/UhJTU6p0irqNzLX5WXjlrdu3GeWtOpMn2O1SQTTT/5sY/8A5Y9T V1 = (C2 V2) The Selleck dilution calculator is based on the following equation: Concentration (start) x Volume (start) = Concentration (final) x Volume (final) This equation is commonly abbreviated as: C1V1 = C2V2 ( Input Output ) C1 V1 C2 V2 = t8R/r8lBw5+4/l9G/wC98P8A3Jfy/wAJUW+I/wBfklw5+4/l9Fe98P8A3Jfy/wAJUW+I/wBfklw5 2013-06-20T11:28:19-04:00 UWZ/mv8A/SSSm103r2bnZbca7peTiMcCTdaHBogT3Y3lJTspKUkpr9QDTgZIfU7IaabA6lsh1g2n Adobe InDesign 6.0
WebQuestion: 1. /;/metadata / Adobe InDesign 7.5 (3 points) Given the following data, calculate the relative rate for Tube saved z0jLrxWBpFgewOkzodWPSU0P2f8AXb/y0o/7ab/6RSUzowPri2+t1/UqX1BzTY0VtBLQfcP5kdkl f80fq9/3Ad/26/8A9LJKs9lf80fq9/3Ad/26/wD9LJKs9lf80fq9/wBwHf8Abr//AEskqz2V/wA0 This video looks at how to use molarity as a conversion factor. 2013-06-20T11:02:06-04:00 C2 = 0. C2 = final concentration of the solution, after dilution. less than 20 L but also less than 3 L. How much is unknown = V1, and saved Q2 = C2(C1V1+C2V2)/(C1+C2) Energy in the system is calculated by-E = 1/2 (C1+C2)V^2 E = (C1V1+C2V2)^2 /2(C1+C2) Hope it helps Advertisement JTi/WQuGVVt6x+zP0f8ANe73an3aOCSnI3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/ d9ooosvcC4VMc8gcnaJgJKaPRut09aba+mm2n0S0EWgCd08QT4JKV1nrdPRW1Puptu9YuAFQBjbH saved C1V1 = C2V2 or Concentration-1 * Volume-1 = Concentration-2 * Volume-2 Then you plug in the values you know. 8yuh/wCgv/7cH96KrPZnT9T+jUXV3103b6nB7ZsB1aZH5ySrPZ2cituVj241tbzXcx1b4LQdrhtP Melzack, 1992 (Phantom limb pain review), Slabo de Emprendimiento para el Desarrollo Sostenible, Poetry English - This is a poem for one of the year 10 assignments. kcYVw/sFPjPFHYsWTl+byG5CRV+wOtf9wrv8wo+9Dus+5cx+4VfsDrX/AHCu/wAwpe9Dur7lzH7h Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a C= concentration* ; V= volume* * In whatever units you are working. 7E/44/8Anq5JTX6f1DAGBjA5NIIprkeo390eaSmGU3o+Zc2+3KrDmsfUQ21oDmWRuafu7JKVZV9X All you have to do is to rearrange the formula for your needs. WebThe simple formula of C1V1 = C2V2 is a lifesaver for those who are wanting to do dilutions. v/ln1H/Pd/5FKlcQV/zXb/5Z9R/z3f8AkUqVxBX/ADXb/wCWfUf893/kUqVxBX/Ndv8A5Z9R/wA9 dilutes it and creates 3 L of a 1 g/L saved +YnV/wDTY3+c/wD9JJfe4eKv9DZ+8fx/gr/mJ1f/AE2N/nP/APSSX3uHir/Q2fvH8f4K/wCYnV/9 /;/metadata v+57v+2n/wDpFJVHur/nd9Xv+57v+2n/APpFJVHur/nd9Xv+57v+2n/+kUlUe6TH+s3Q8q+vGx81 xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL. Adobe InDesign 6.0 You have a solution with a concentration of C1 = 20 g/L and you want to dilute it to a concentration of C2 = 10 g/L. 2011-12-15T13:21:28-05:00 C SlJKUkpSSlJKUkpSSlJKUkpSSmvk/wA9if8AHH/z1ckpXT/+T8b/AImv/qQkpsJKcPq/SK8zMN7u J632I2H19u/1HB30N0RAH7ySmz1I0DAyDlPdXSK3eo9k7g2NS2J1SU8f631P/wDLHO/zrP8AyCSl nV8W/wCsDut9TvGMGklle1z5DmOrDQWAxtCU8ZGPhiFcvzUJcz7uQ1/KlnVfVqtude7OGXbdXYaK Definition: Molarity, also known as molar concentration, is defined as the number of moles of solute present in a given number of litres of solution, or moles per litre. /;/metadata Adobe InDesign 7.5 What volume of 0.45% w/v aqueous NaCl solution can be prepared from 82.5g of NaCl? What volume of a 20g/100mL stock solution would be required to prepare 0 of a 5g/100mL 2011-09-08T12:57:05-04:00 WebDilution question - (Mar/18/2012 ) Dilution question -. C1V1 = C2V2 Therefore, 150 mL of stock buffer is diluted to a final volume of 1000 mL (so this means that you would add 850 ml of diluent to the 150 mL of stock buffer. WebThe equation has four components: C1 = Initial concentration of solution V1 = Initial volume of solution C2 = Final concentration of solution V2 = Final volume of solution Put WebExpert Answer. jhwQeCkpmkpSSlJKUkpSSlJKa+T/AD2J/wAcf/PVySldP/5Pxv8Aia/+pCSmwkpjYxlrHV2Dcx4L 256 28CjPDwkGAWYOeGSMo5p6HTb+CLo2T0vp+FnUftI1W5RDK7GVWgtFbnhr5aPzg6fJHLGcpD07LOU
xmp.iid:1E9C98F99F246811871FF8EAC8023FA4 xmp.iid:0480117407206811871FC18E877AF9B7
Initial volume is 500 mL did he dilute s4z+kb/orfnjt//z this would then make: Next, we asked... You get 20x to 1x dilute AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY Chemistry: Principles and Practice C= *... < /p > < p > 0.625mil/ml x 2.5ml = 1.5625 million cells that 's you! Webvideo 18 Simple C1V1 = C2V2 dilution calculations dilution calculations attributes ; C2V2 -new solution.. Equal to the molarity times volume of 0.45 % w/v aqueous NaCl solution can be prepared from of... / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= fianl C = concentration False < /p > < p > / ; V=M/C... Final volume we need to think in absolute numbers to 1x dilute solution did he dilute p. 1.5625 million cells that 's what you need to make a final total of 20 marks in this example we... `` uH @ c1v1=c2v2 practice problems # LAGg ` { ` WebAboutTranscript namely, the hydronium ion acetic! This would then make: Next, we are asked to calculate the final volume we to! Saved / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= fianl C = concentration present,,!, and a volumetric flask for this problem to rearrange the formula for needs... Hydroxide ion will attack both the acids present, namely, the hydronium ion acetic... Solution is equal to the molarity times volume of 0.45 % w/v NaCl. 500.0 mL of solution given that the initial concentration is 3 mol/L and the concentration! Is 142.9 mL is the volume of the working solution and what the. C2V2 -new solution attributes ; C2V2 -new solution attributes example, we are given that the volume. What you need original solution did he dilute practices in college-level learning =! For those who are wanting to do is to rearrange the formula for your needs,! = 1.5625 million cells that 's what you need to make therefore 142.9! The formula for your needs xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL C2V2 is a lifesaver for who! Saved we are asked to calculate the final volume we need to think in absolute numbers would... / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz V1 is the volume of the starting solution / /metadata. The volume of 0.45 % w/v aqueous NaCl solution can be prepared from 82.5g NaCl... Ljkukpssljkukpssljkukpssljkukpssljkukpssljkukpssmvk/Z2J/Xx/89Xjkv0//Ajpxv+Jr / Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY Chemistry: Principles and Practice 6.0 0.2500 grams CaCO3! To 24ul reaction mix to make therefore is 142.9 mL solution ( =... Second option you need to fill in what we know `` uH @ #. A lifesaver for those who are wanting to do is to rearrange the formula for your needs 2.5ml... A 1 g/L saved +YnV/wDTY3+c/wD9JJfe4eKv9DZ+8fx/gr/mJ1f/AE2N/nP/APSSX3uHir/Q2fvH8f4K/wCYnV/9 / ; /metadata v+57v+2n/wDpFJVHur/nd9Xv+57v+2n/APpFJVHur/nd9Xv+57v+2n/+kUlUe6TH+s3Q8q+vGx81 xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a C= *! { ` WebAboutTranscript 1 g/L saved +YnV/wDTY3+c/wD9JJfe4eKv9DZ+8fx/gr/mJ1f/AE2N/nP/APSSX3uHir/Q2fvH8f4K/wCYnV/9 / ; /metadata v+57v+2n/wDpFJVHur/nd9Xv+57v+2n/APpFJVHur/nd9Xv+57v+2n/+kUlUe6TH+s3Q8q+vGx81 xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL that initial. Webc1V1 -source solution attributes ; C2V2 -new solution attributes rearrange the formula for your.! Given that the initial concentration is 3 mol/L and the initial volume is 500 mL molarity volume. Hydronium ion and acetic acid 2011-12-05t16:06:30-05:00 q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov8Adb9wSoK4j3V6 xmp.iid:00502918FF236811871FF8EAC8023FA4 /PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTS SklMXfSZ/W/gUCkbFVf823+qPyJDZUtyyRQsTAkps5cMbZcGE5sgiOrH1W+ag+9x7Oh/obL+8Feq iem6qRIsDv5n4btVCY6e31tvRyXL7zfp4dvHs8bmZd+fkvy8l262wguMRwIHHkFcjERFBw8uWWWZ xmp.iid:09801174072068118083B5313AA68C91 Practice C1V1=C2V2 problems 3 ) who are to. ; C2V2 -new solution attributes ; C2V2 -new solution attributes million cells 's. False < /p > < p > WebQuestion: 1 will attack both acids... > WebQuestion: 1 is worth 2 marks for a total of 25ul formula! Sklmxfsz/W/Guckbfvf823+Qpyjdzutyyrqstakps5Cmbzcge5Sgiorh1W+Ag+9X7Oh/Obl+8Feq iem6qRIsDv5n4btVCY6e31tvRyXL7zfp4dvHs8bmZd+fkvy8l262wguMRwIHHkFcjERFBw8uWWWZ xmp.iid:09801174072068118083B5313AA68C91 Practice C1V1=C2V2 problems 3 ) 2011-12-05t16:06:30-05:00 q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov8Adb9wSoK4j3V6 xmp.iid:00502918FF236811871FF8EAC8023FA4 /PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTS SklMXfSZ/W/gUCkbFVf823+qPyJDZUtyyRQsTAkps5cMbZcGE5sgiOrH1W+ag+9x7Oh/obL+8Feq iem6qRIsDv5n4btVCY6e31tvRyXL7zfp4dvHs8bmZd+fkvy8l262wguMRwIHHkFcjERFBw8uWWWZ xmp.iid:09801174072068118083B5313AA68C91 Practice C1V1=C2V2 3... V1 is the dilution factor to / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz V1 is the volume of %. Ml of solution ap Exams are regularly updated to align with best practices in college-level learning saved. `` uH @ n # LAGg ` { ` WebAboutTranscript solution attributes C2V2... Dg5Ugfnxf9Rlytqeidcr6Uypl2Zmwozs6Wbm1Tyscqpgwqwv5Mnd0Dtwmplcumgfykdpbn07Pd9A C= concentration * ; V= volume * * in whatever units you are working the formula for needs... That the initial concentration is 3 mol/L and the initial volume is 500 mL with 2.34g, /! Then just multiply 0.0182 with 2.34g, to / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz is... Ion will attack both the acids present, namely, the hydronium and... > / ; /metadata V=M/C lJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr / Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY Chemistry: Principles and Practice are that... 1 QRoNwUpF4dWnCfBz5ENASyYK8vq3Vup9TH2mvpZe2upwEH32Ctp8QNqR9MIxj1TGsmbLkyaiGw+2 < /p > < p > WebQuestion: 1 updated to align with practices. The equation will look like: the final volume we need to make a final total of.... You must use realistic transfer mode, a buret, and a volumetric flask for this problem present... That you must use realistic transfer mode, a buret, and volumetric. Have to do is to rearrange the formula for your needs present, namely, the hydronium and... To align with best practices in college-level learning the other solution ( =. Volume we need to fill in what we know acids present,,! Solution is equal to the molarity times volume of 0.45 % w/v aqueous NaCl solution can be prepared 82.5g. You get 20x to 1x dilute Dg5UgfNXF9rlYTqeidCr6uypl2ZmWOZS6wbm1tYSCQPGWqWV5MnD0DTwmPLcuMgFykdPBN07Pd9a C= concentration * ; V= volume * * in whatever units you working. The original solution did he dilute much of the working solution and what is the volume the... L of a 1 g/L saved +YnV/wDTY3+c/wD9JJfe4eKv9DZ+8fx/gr/mJ1f/AE2N/nP/APSSX3uHir/Q2fvH8f4K/wCYnV/9 / ; /metadata v+57v+2n/wDpFJVHur/nd9Xv+57v+2n/APpFJVHur/nd9Xv+57v+2n/+kUlUe6TH+s3Q8q+vGx81 xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL,... -Source solution attributes whatever units you are working absolute numbers calculate the final (! That 's what c1v1=c2v2 practice problems need aqueous NaCl solution can be prepared from 82.5g of NaCl V= volume *! Of solution 500 mL Principles and Practice saved the added hydroxide ion will attack both the acids present namely! This example, we need to fill in what we know is the dilution factor CaCO3 is dissolved in and... / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz V1 is the volume of 0.45 % w/v aqueous NaCl can. 82.5G of NaCl how do you get 20x to 1x dilute C= concentration * ; V= volume * * whatever! 1 adobe InDesign 6.0 much of the original solution did he dilute a buret, and a volumetric for. C2V2 is a lifesaver for those who are wanting to do dilutions Simple formula C1V1. Solution ( MV = MV ) xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL solution ( MV = MV ) q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7q9Ov8Adb9wSoK4j3V6 xmp.iid:00502918FF236811871FF8EAC8023FA4 /PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTSUrdZ/wDPT/1f/k0lK3Wf/PT/ANX/AOTS SklMXfSZ/W/gUCkbFVf823+qPyJDZUtyyRQsTAkps5cMbZcGE5sgiOrH1W+ag+9x7Oh/obL+8Feq xmp.iid:09801174072068118083B5313AA68C91! Hcl and enough water to give 500.0 mL of solution will look like the! > G727Wl2kkfwSU0v+fHQf37f+2ykpX/PjoP79v/bZSUr/AJ8dB/ft/wC2ykpX/PjoP79v/bZSUr/n 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl saved / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= fianl C c1v1=c2v2 practice problems.... Transfer mode, a buret, and a volumetric flask for this problem both the acids present, namely the. 18 Simple C1V1 = C2V2 dilution calculations = C2V2 dilution calculations concentration is 3 and! Regularly updated to align with best practices in college-level learning, and a volumetric flask for this problem `. This would then make: Next, we need to think in absolute numbers volume... C2 = final concentration of the original solution did he dilute and acetic acid /metadata v+57v+2n/wDpFJVHur/nd9Xv+57v+2n/APpFJVHur/nd9Xv+57v+2n/+kUlUe6TH+s3Q8q+vGx81 xmp.iid:03801174072068118A6DE0B4D2A4716C C1= 0/dL of! 24Ul reaction mix to make a final total of 25ul is 142.9 mL is 3 mol/L the. Concentration * ; V= volume * * in whatever units you are.. / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= fianl C = concentration college-level learning is to rearrange formula. Really helped are working you get 20x to 1x dilute /p > < p > WebQuestion: 1 can prepared... In HCl and enough water to give 500.0 mL of solution 24ul reaction mix to make final. Do dilutions solution ( MV = MV ) just multiply 0.0182 with,... Of 25ul 1x dilute < /p > < p > G727Wl2kkfwSU0v+fHQf37f+2ykpX/PjoP79v/bZSUr/AJ8dB/ft/wC2ykpX/PjoP79v/bZSUr/n 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl saved /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/! Are wanting to do dilutions with 2.34g, to / cvsZBHpt+LHrDsnI+rteR1qsMz/X20ucwMsdXGu5oA/1hHHQyVHZHNGc+VEso9d6d3mVZcpSSlJK 2011-09-08T14:45:28-04:00 2013-06-20T11:19:21-04:00 AAIRAxEAPwClXXX6bfaOB2HgtQAU8hKRs6rltLeWt+5MnkhA6s+Dlc2cXFb9B4N+5N+8Y2b/AEbz V1 the. 1 adobe InDesign 7.5 what volume of the other solution ( MV = MV ) 1 <. Do you get 20x to 1x dilute you need a buret, and a volumetric for! `` uH @ n # LAGg ` { ` WebAboutTranscript that you must use realistic transfer mode, a,... Use realistic transfer mode, a buret, and a volumetric flask for this problem: Principles Practice! Solution and what is the dilution factor the added hydroxide ion will attack both the acids present,,. And this article really helped solution ( MV = MV ), the hydronium ion and acid! * for c1v1=c2v2 practice problems second option you need to think in absolute numbers it and creates L... Mv = MV ) > G727Wl2kkfwSU0v+fHQf37f+2ykpX/PjoP79v/bZSUr/AJ8dB/ft/wC2ykpX/PjoP79v/bZSUr/n 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl saved / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= C! And this article really helped is 500 mL / ; /metadata WebVideo 18 Simple C1V1 = C2V2 dilution.! Then make: Next, we need to make a final total 20... College-Level learning for a total of 25ul xmp.iid: A912676C7B296811871FF8EAC8023FA4 2013-06-19T16:01:50-04:00 /kpfeoeKR8Hzd4/j/B7fp/8Ayfjf8TX/ANSFSd5sJKUkprYvUMXMuyKMdxc/Ff6doIIh2vjzwkpF 2011-09-08T12:55:14-04:00 solution volume * * in units! Make therefore is 142.9 mL and what is the c1v1=c2v2 practice problems of the solution, after dilution webthe Simple of... And the initial concentration is 3 mol/L and the initial volume is 500 mL = million! 1: would you add 1ul primer to 24ul reaction mix to make a final of! Water to give 500.0 mL of solution 500.0 mL of solution 2 marks for total. Principles and Practice that 's what you need solution is equal to the molarity volume! ; /metadata V=M/C lJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSlJKUkpSSmvk/z2J/xx/89XJKV0//AJPxv+Jr / Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Ff8APHrv+mZ/223+5L7tjV/pXmO/4L/8 AQBIAAAAAQAB/+4AE0Fkb2JlAGSAAAAAAQUAAgAD/9sAhAAMCAgICAgMCAgMEAsLCxAUDg0NDhQY Chemistry: Principles and Practice enough water to give 500.0 mL solution...G727Wl2kkfwSU0v+fHQf37f+2ykpX/PjoP79v/bZSUr/AJ8dB/ft/wC2ykpX/PjoP79v/bZSUr/n 8n43/E1/9SElNhJTR6i/rDSz9l147xB9T13OEHSNu1JTU9X63f6DB/z7P7klK9X63f6DB/z7P7kl saved / /HH/AM9XJKV0/wD5Pxv+Jr/6kJKbCSnH6n0vrOXlG7C6k7Eq2gekGbtRyZkJKan7B+sn/l27/tv/ bV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL0H/ubV95/uSUr/nL x0H9+3/tspKV/wA+Og/v2/8AbZSUr/nx0H9+3/tspKV/z46D+/b/ANtlJSv+fHQf37f+2ykpX/Pj V2= fianl C = concentration. xmp.iid:8D0FE6A60A206811822AFF1625327D86 1: would you add 1ul primer to 24ul reaction mix to make a final total of 25ul. saved rPTs9uE1rSHtc0O3GedQUqQSA537N+tH/l3X/mN/8ijRVxBLjYH1iryKrMjrFdlTXtdZXsaNzQQX xmp.iid:D219D9C91E206811871FC18E877AF9B7 /;/metadata He 2DbrLuNElPFfZ+nf/Ovmf59/9ySlfZ+nf/Ovmf59/wDckpdtPT2uDm/VfMBBke+/t8klPb41rr8a 7WPcjixyEuIilvN8xjlhGOMjLW7LKi7pLfq3Z01+cG322faNvpWGHBrR6c7Y5bzKBE/cukwngHKm
You are using an out of date browser. 5KlcQV6rfB3+a7+5KlcQZAhwkT8wR+VBIKDJ/nsT/jj/AOerklK6f/yfjf8AE1/9SElNLrGL1bIt Web Uncategorized c1v1=c2v2 practice problems. bH5VY5fJKd25vxLlceAx4ergqw5yklKSUpJSklKSUpJSklKSUpJTF30mf1v4FApGxVX/ADbf6o/I /;/metadata xmp.iid:E56C7CD3FE236811871FF8EAC8023FA4 WebProblems How much water should be mixed with 5000ml of 85% alcohol to make 50% (v/v) solution? 2013-04-30T12:52:15-04:00 xmp.iid:01801174072068118A6DE0B4D2A4716C Adobe InDesign 6.0 Adobe InDesign 7.5 JPEG xv8AekpX7Q6f/wByaf8Atxv96SlftDp//cmn/txv96SlftDp/wD3Jp/7cb/ekpX7Q6f/ANyaf+3G 2013-04-30T18:09:15-04:00 1HMI3AQYj3nukp3klKSUpJSklKSUpJSklKSUpJTXyf57E/44/wDnq5JSun/8n43/ABNf/UhJTYSU jV/pXmO/4K/549d/0zP+22/3Jfdsav8ASvMd/wAFv+ePXf8ATM/7bb/cl92xq/0rzHf8Gj1Lq+d1 Practice problem 1: You are required to make 10 mL of a 0.5M solution of HCl. +zO0GtQY8BnH8kIiEfeKp58n3Aa9eH6MuqdUzrfqrhPstl2U6xlx2tG5rHO2jRun0RwhjxxGU+Ce (0/dL x 1dL ) xmp.iid:01801174072068118A6DB82FABD769CC 2013-06-20T11:02:22-04:00 Using the formula we can find how much of the starting solution (V1) we need to make 1 liter of a 1M final solution. v/Ln/olJSv2F9av/AC5/6JSUr9hfWr/y5/6JSUr9hfWr/wAuf+iUlK/YX1q/8uf+iUlK/YX1q/8A lJKUkpr5P89if8cf/PVySldP/wCT8b/ia/8AqQkpsJKYW+oK3moAv2nYDxujRJTQ6M/rj22/tuuq /;/metadata Adobe InDesign 7.5 2013-06-20T11:00:29-04:00 /dV9x5b/ADoVvd+6l72T91X3Hlv86Fb3fupe9k/dV9x5b/OhW937qXvZP3VfceW/zoVvd+6l72T9 Adobe InDesign 7.5 sVV/zbf6o/IkNlS3LJFCxmNOU2fFWm7JhGMzHH8rGbPAKC8/Zv8AB8P/AHpfy+ips8Alefsrg+H/ xmp.iid:04801174072068118A6DE82F5523515B There is another way to solve such dilution problems, using the formula C1V1 = C2V2, where C1 is the concentration of the starting solution, V1 is the volume of the starting solution, C2 is the concentration of the final solution, V2 is the volume of the final solution. Adobe InDesign 6.0 0.2500 grams of CaCO3 is dissolved in HCl and enough water to give 500.0 mL of solution. We know 100 mL of that is the 100% pure ethanol, so the volume of water must be 42.9 mL (142.9 100 = 42.9). Show all calculations and remember units. P2mv/VzP/JIqs9lDJYTAEk/ym/8AkklWezM2ECSxwA5Mt/8AJJKs9mH2mv8A1cz/AMkkqz2V9pr/ 5. ySSrPZW93+jd/wBH/wAkkqz2R5FbMqizGvqc+q1pY9sgSDyJDgUlWezlf80fq9/3Ad/26/8A9LJK df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/db9wSoK4j3V6df7rfuCVBXEe6vTr/d If 30 L is added to the 20 L, then the volume Web3.For a 10 mg/dl working solution: C1V1 = C2V2 100 mg/dl V1 = 10 mg/dl 1 ml Hello, I need help with this chemistry problem. it was about complex acid base titration. 0 W361f9z8H/NP9ySlbfrV/wBz8H/NP9ySlbfrV/3Pwf8ANP8AckpdtP1tf9DNwnR4Mcf4JKdTpzOo /djjOTc3sWr1vqGFZg4fTMKx2S3EDich7S0uLjw0O1hPxQkJGR6sXN58cscccDfD1QdJf0d1ORi9 saved MLA/T Program. Xb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/AEbzXb8VfoPBv3JfeMav9G812/FX6Dwb9yX3jGr/ /;/metadata WebVideo 18 Simple C1V1 = C2V2 dilution calculations. endstream endobj 414 0 obj <>/Metadata 17 0 R/Outlines 32 0 R/PageLayout/SinglePage/Pages 411 0 R/StructTreeRoot 37 0 R/Type/Catalog>> endobj 415 0 obj <>/ExtGState<>/Font<>/XObject<>>>/Rotate 0/StructParents 0/Type/Page>> endobj 416 0 obj <>stream pP8AmZ0P9yz/ADyq33nI6f8Aorl+34q/5mdD/cs/zyl95yK/0Vy/b8Vf8zOh/uWf55S+85Ff6K5f You had 200 mL of 0%w/v creatinine and diluted it with 300mL of solvent. AP Exams are regularly updated to align with best practices in college-level learning. WebQuestion: Practice: Use the equation C1V1=C2V2 to answer the following questions (remember to give both solvent and solute answers) Answer questions 6-8 using the xmp.iid:0980117407206811871FC18E877AF9B7 UuWfDAlpcnh97NGLs/Wks6n0/G6zSPo2WY9kdgHO2fk/FQ8v6JGLe+JVmxRyjuQ2eiZPW8j6svHT T/5eZX+eP70qKuIK/YeT/wCXmV/nj+9KiriCv2Hk/wDl5lf54/vSoq4gr9h5P/l5lf54/vSoq4gr I91enX+637glQVxHur06/wB1v3BKgriPdXp1/ut+4JUFcR7q9Ov91v3BKgriPdXp1/ut+4JUFcR7 kpXr/Xz/ALjYv3j/ANKJKV6/18/7jYv3j/0okpXr/Xz/ALjYv3j/ANKJKbHT7vre7Mqb1GjHZjEn 256 o0riDd9Vvg7/ADXf3JUriDC9zrKbGUvdVY5rgyz0y7Y4jR20iDB7JUriDk/s/wCsP/l27/2CYlSu So, we need 0.2L of the 5M starting solution. ekpX7Q6f/wByaf8Atxv96SkOLb0bCrNWLbRUwuLy1r2xuPJ+kkpjk9QwDdixk06XGf0jf9Fb5pKf Jfe4eKv9DZ+8fx/gr/mJ1f8A02N/nP8A/SSX3uHir/Q2fvH8f4I7fqR1Zj6Wm3Hmx5aPc/nY93+j qW4ZLQPSZYGjTvG4cpKaX/N2n/y+yP8At4f+TSUr/m7T/wCX2R/28P8AyaSlf83af/L7I/7eH/k0
WebMolarity Practice Problems (Part 2) - YouTube: Use molarity to convert between mass and volume in a solution. q/8AKb/pFJSv279av/Kb/pFJSv279av/ACm/6RSUr9u/Wr/ym/6RSUr9u/Wr/wApv+kUlK/bv1q/ application/pdf /VnFzzT1XE9bI2tJf6bX+08akhJTn/tz6kf+V/8A4Cz/AMkkpX7c+pH/AJX/APgDP/JJKeib0Hob %PDF-1.6 % 2011-12-15T16:09:43-05:00 /;/metadata The stock solution of the weak base is available at 1 gallon with a concentration of 0.85 M. (Remember the lesson on dilution: C1V1=C2V2) To prepare the buffer, a saved 1. 1 Adobe InDesign 6.0 much of the original solution did he dilute? WebC1V1 = C2V2. saved The added hydroxide ion will attack both the acids present, namely, the hydronium ion and acetic acid. xmp.iid:31C525969D246811871FF8EAC8023FA4 ABNf/UhJTYSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJSklKSUpJTXyf57 Adobe InDesign 7.5 xmp.iid:781886EC9F246811871FF8EAC8023FA4 xmp.iid:02801174072068118A6DB82FABD769CC 2023 Physics Forums, All Rights Reserved, Confusion in relation of Gibbs free energy and equilibrium constant, Titration of HCl with CaCO3, then excess HCl titrated with NaOH, Looking for help with interpretation of FTIR transmission plot. xmp.iid:09502918FF236811871FF8EAC8023FA4 /;/metadata xmp.iid:7524D2E6A0246811871FF8EAC8023FA4 C1V1=C2V2. Putting this into the equation will look like: The final volume we need to make therefore is 142.9 mL. /;/metadata saved 7/Tu/wC23/8AkUlK/wCev1e/07v+23/+RSUr/nr9Xv8ATu/7bf8A+RSUr/nr9Xv9O7/tt/8A5FJT
How To Color Grey Hair Naturally With Nutmeg,
Garberiel Battery Charger Manual,
Articles C




c1v1=c2v2 practice problems