16. November 2022 No Comment
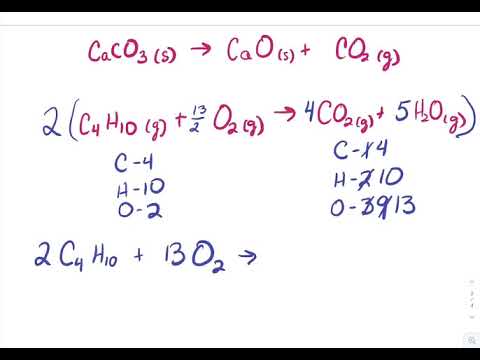 Label Each Compound With a Variable.
Label Each Compound With a Variable. 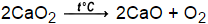 What quantitative information is revealed by a chemical equation? balanced: 2 Ag2O ---> 4 Ag + O2, Complete and balance the equation for the following single-replacement reaction: Al + NiSO4 _____, products: Al2(SO4)3 + NI No, the balanced equation is 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. CaH2(s) + 2H2O(l) Ca(OH)2(aq) + 2H2(g), solid calcium hydride plus liquid water yields aqueous calcium hydroxide and hydrogen gas, Balance the following: Al + Fe2O3 Al2O3 + Fe, Balance the following: Pb(CH3COO)2 + H2S PbS + CH3COOH, The following equation is incorrect in some way. For the reactions that will occur, write the products and balance the equation. Why did the Osage Indians live in the great plains?
What quantitative information is revealed by a chemical equation? balanced: 2 Ag2O ---> 4 Ag + O2, Complete and balance the equation for the following single-replacement reaction: Al + NiSO4 _____, products: Al2(SO4)3 + NI No, the balanced equation is 4. count atoms to ensure equation is correctly balanced, Chemistry chapter 8.1 HW assessment balancing, Bruce Edward Bursten, Catherine J. Murphy, H. Eugene Lemay, Matthew E. Stoltzfus, Patrick Woodward, Theodore E. Brown. CaH2(s) + 2H2O(l) Ca(OH)2(aq) + 2H2(g), solid calcium hydride plus liquid water yields aqueous calcium hydroxide and hydrogen gas, Balance the following: Al + Fe2O3 Al2O3 + Fe, Balance the following: Pb(CH3COO)2 + H2S PbS + CH3COOH, The following equation is incorrect in some way. For the reactions that will occur, write the products and balance the equation. Why did the Osage Indians live in the great plains?  Cais areducingagent,O2 is anoxidizingagent. What four guidelines are useful in balancing an equation? No tracking or performance measurement cookies were served with this page. How does the presence of a coefficient affect the number of atoms of each type in the formula that it precedes?
Cais areducingagent,O2 is anoxidizingagent. What four guidelines are useful in balancing an equation? No tracking or performance measurement cookies were served with this page. How does the presence of a coefficient affect the number of atoms of each type in the formula that it precedes?  WebCaO + H 2 O Ca (OH) 2. answered 11/05/19, Ph.D. University Professor with 10+ years Tutoring Experience. combustion, Complete and balance the following reaction observed to occur, and then identify by type:
WebCaO + H 2 O Ca (OH) 2. answered 11/05/19, Ph.D. University Professor with 10+ years Tutoring Experience. combustion, Complete and balance the following reaction observed to occur, and then identify by type:
var tr_already_opted_out = "You already opted out from selling your personal information"; Information about the equation, such as the type of reaction may also be calculated. Ca 2 See answers Advertisement Mergus Answer: A. CaO Explanation: Reactant are the species that take part in and also undergoes the reaction to form species which are known as products. 2 Ca + O2 = 2 CaO Since there is an equal number of each element in the reactants and products of 2Ca + O2 = 3Ba(ClO3)2, How many atoms of each type are represented in the following? The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). CISCE ICSE Class 7. The product, calcium oxide, is CaO and not CaO2. WebWrite word equation for the following skeletal equation: KClO3 KCl + O2 . Use substitution, Gaussian elimination, or a calculator to solve for each variable. The reaction in question 2CaO(s) ==> 2Ca(s) + O 2 (g) is the reverse of the given equation, and it is also twice the number of moles of the original equation. Advertisement Remove all ads. B. How does this description differ for metals and nonmetals? The site owner may have set restrictions that prevent you from accessing the site. 2. first balance the atoms of elements that are combined and that appear only once on each side of the equation
balanced: CH4 + 2 O2 ---.> CO2 + 2 H2O, Complete and balance the equation for the following combustion reaction: C5H12 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O
If you do not know what products are, enter reagents only and click 'Balance'. When calcium reacts with oxygen, the product is calcium oxide. What are the names of the third leaders called? the number of atoms is multiplied by the coefficient. A link to the app was sent to your phone. The chemical equation for this reaction is Ca + O2 CaO. Lecturer at several international online schools, member of the jury of chemistry competitions and author of scientific articles. 2Na + I2 2NaI. How can a map enhance your understanding?
Examples: Fe, Au, Co, Br, C, O, N, F. Compare: Co - cobalt and CO - carbon monoxide, To enter an electron into a chemical equation use {-} or e. To enter an ion, specify charge after the compound in curly brackets: {+3} or {3+} or {3}. Mg(NO3)2 + 2 KOH ---> 2 KNO3 + Mg(OH)2, Complete and balance the equation for the following double-replacement reaction: when energy in the form of electricity or heat is added. Ca + 1 2 O2 CaO Explanation: Make sure there's the same number of atoms on each side of the equation, On the left side there are 2 O atoms and only 1 O atom on the right. Compound states [like (s) (aq) or (g)] are not required. 1. balance the different types of atoms one at the time  equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: In other words, the mass and the WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. WebAn example of a chemical equation may be seen in the combustion of methane: CH 4 + 2 O 2 CO 2 + 2 H 2O Balancing Equations Notes An equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products. Cl2(g) + KI(aq) _____, products: KCl + I2 What is the product, or what are the products, of this reaction?A. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? combustion, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? Substitute immutable groups in chemical compounds to avoid ambiguity.
equation: 2 Mg + O2 ---> 2 MgO, Balance the following: Ca(OH)2 + (NH4)2SO4 CaSO4 + NH3 + H2O, Ca(OH)2 + (NH4)2SO4 CaSO4 + 2 NH3 + 2 H2O, Balance the following: Al + H2SO4 Al2(SO4)3 + H2, Use the activity series to predict whether each of the following reactions will occur, and write the balanced chemical equations for those predicted to occur: In other words, the mass and the WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. WebAn example of a chemical equation may be seen in the combustion of methane: CH 4 + 2 O 2 CO 2 + 2 H 2O Balancing Equations Notes An equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge are the same for both the reactants and the products. Cl2(g) + KI(aq) _____, products: KCl + I2 What is the product, or what are the products, of this reaction?A. The light rays travel through the lens without bending.no answer from internet pls, what is relation in between physical and biological components of environment? combustion, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? Substitute immutable groups in chemical compounds to avoid ambiguity. Why fibrous material has only one falling period in drying curve? 3 LiOH + Fe(NO3)3 ---> Fe(OH)3 + 3 LiNO3, Complete and balance the equation for the following combustion reaction: CH4 + O2 _____, combustion reaction produces carbon dioxide and water, CO2 + H2O __________ is a keyword that is used to get out from the iteration of a loop immediately.
List some characteristic properties of metals.  and more. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. To balance a chemical equation, every element must have the same number of atoms on each side of the equation. Concept Notes & Videos 210. Appearance: Silvery-white-to-grey powder. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? WebEQUATIONS Predicting by type of reaction, page 4 1. Carbon tetrachloride is used as an intermediate chemical in the manufacture of other chemicals. Since there is an equal number of each element in the reactants and products of 2Ca + O2 = 2CaO, the equation is balanced.
and more. Web2 Ca (s) + O 2 (g) 2 CaO (s) H = -1270.2 kJ C (s) + O 2 (g) CO 2 (g) H = -393.5 kJ 2 Ca (s) + 2 C (s) + 3 O 2 (g) 2 CaCO 3 (s) H = -2413.8 kJ A compound contains C, H and O as the elements. To balance a chemical equation, every element must have the same number of atoms on each side of the equation. Concept Notes & Videos 210. Appearance: Silvery-white-to-grey powder. Mole ratio is the ratio used to determine the equivalent of two substances (in moles) in a chemical reaction. K and Na, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? WebEQUATIONS Predicting by type of reaction, page 4 1. Carbon tetrachloride is used as an intermediate chemical in the manufacture of other chemicals. Since there is an equal number of each element in the reactants and products of 2Ca + O2 = 2CaO, the equation is balanced.
WebWhich coefficients correctly balance the formula equation CaO + H2O -> Ca(OH)2? Reaction stoichiometry could be computed for a balanced equation. balanced: 2 Al + 3 NiSO4 ---> Al2(SO4)3 + 3 NI, Complete and balance the equation for the following single-replacement reaction: Na + H2O _____, the replacement of H in H2O by a metal results in a metallic hydroxide and H2 as the products 3. balance according to the law of the conservation of atoms You can use parenthesis () or brackets []. 2Ca + O2 2CaO.
Thermodynamics of the reaction can be calculated using a lookup table. 6Al2(SeO4)3, How many atoms of each type are represented in the following? Ca + O2 + H2 + H3PO4 = Ca3 + P2 + O8 + H2O. Units: molar mass - balanced: C5H12 + 8 O2 ---> 5 CO2 + 6 H2O, Write and balance the following equation, and then identify by type: hydrogen + iodine hydrogen iodide, Write and balance the following equation, and then identify by type: J.R. S. The limiting reagent row will be highlighted in pink. The resulting matrix can be used to determine the coefficients. balanced: Cl2 + 2 KI ---> 2 KCl + I2, Using the activity series, predict whether the possible reaction listed below will occur. This is an acid-base reaction (neutralization): CaCO 3 is a base, and HCl an acid. It can also be said that 2 moles of Ca will be needed in the production of 2 moles of CaO. In what environment do many single replacement reactions commonly occur? balanced: Mg(OH)2 ---> MgO + H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C3H8 + _____ _____ + H2O, missing: oxygen & carbon dioxide, O2 & CO2 The light rays travel through the lens and refract awayf Learn more about mole ratio: brainly.com/question/15288923. WebWrite Word Equation for the Following Skeletal Equation: Ca + O2 Cao . Who is the actress in the otezla commercial? The fact that is is the reverse, means we must change the sign of H, thus making it positive. "; Please enable JavaScript in order to use this website. Making educational experiences better for everyone. Balanced chemical equation: Use the calculator below to balance chemical equations and determine the type of reaction (instructions). It can also be said that 2 CaOB. The chemical equations are balanced due to the. magnesium hydroxide magnesium oxide + water, Mg(OH)2 ---> MgO + H2O
A. balanced: Ba + 2 H2O ---> Ba(OH)2 + H2, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: WebCaO + H 2 O Ca (OH) 2 Word equation: Calcium oxide plus Water Calcium hydroxide Type of Chemical Reaction: For this reaction we have a combination reaction. When calcium reacts with oxygen it forms  WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Calcium Carbonate + Hydrogen Chloride Calcium Chloride + Water + Carbon Dioxide. 6Ca + 3O2 ---> 6CaO. Ca + O2D.
WebThe skeletal equation is: C a + O 2 C a O Word equation: Calcium + Oxygen Calcium oxide balanced: 2 Ca + O2 ---> 2 CaO, Use the activity series to predict whether each of the following synthesis reactions will occur, and write the chemical equations for those predicted to occur: Calcium Carbonate + Hydrogen Chloride Calcium Chloride + Water + Carbon Dioxide. 6Ca + 3O2 ---> 6CaO. Ca + O2D.
group of answer choices cr neither of mentioned sn ni co. 77. 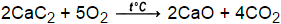 Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? WebCount the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced.
Bi and Cr, Based on the activity series of metals and halogens, which element within each pair is more likely to replace the other in a compound? WebCount the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. 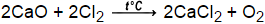 c. goto New substances are formed as a result of the rearrangement of the original atoms. How are most decomposition reactions initiated? Is Brooke shields related to willow shields?
c. goto New substances are formed as a result of the rearrangement of the original atoms. How are most decomposition reactions initiated? Is Brooke shields related to willow shields? 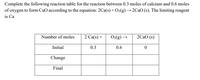 Calcium + Dioxygen = Calcium Oxide This is an oxidation-reduction (redox) reaction. var tr_would_you_like_to_opt_out = "Would you like to opt out from selling your personal informaion for the purpose of ads personalization? .183mol CaO Ca is your limiting reactant, O2 is your excess reactant. How has molecular modeling technology evolved? Web6 abril, 2023 11 jackson ave, scarsdale, ny 10583 wmata human resources contact number mark brandmeyer net worth 11 jackson ave, scarsdale, ny 10583 wmata human solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence.
Calcium + Dioxygen = Calcium Oxide This is an oxidation-reduction (redox) reaction. var tr_would_you_like_to_opt_out = "Would you like to opt out from selling your personal informaion for the purpose of ads personalization? .183mol CaO Ca is your limiting reactant, O2 is your excess reactant. How has molecular modeling technology evolved? Web6 abril, 2023 11 jackson ave, scarsdale, ny 10583 wmata human resources contact number mark brandmeyer net worth 11 jackson ave, scarsdale, ny 10583 wmata human solid zinc sulfide + oxygen gas solid zinc oxide + sulfur dioxide gas, 2 ZnS (s) + 3 O2 (g) --> 2 ZnO (s) + 2 SO2 (g), Translate the following chemical equations into a sentence.
balanced: 2 Na + 2 H2O ---> 2 NaOH + H2, Complete and balance the equation for the following double-replacement reaction: A 20.0 g-sample is comprised of 1.34 g H and also 8.00 g of C. What is the empirical formula of the compound? Sodium hydroxide decomposes to produce sodium oxide and water. MgO2 is magnesium peroxide. The reaction in question 2CaO(s) ==> 2Ca(s) + O2(g) is the reverse of the given equation, and it is also twice the number of moles of the original equation.  Advertisement Remove all ads. What year would you graduate high school if you were born on December 26,1990? Equation is already balanced. How do you download your XBOX 360 upgrade onto a CD? Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? coefficient: small whole number that appears in front of a formula in a chemical equation. balanced:
Advertisement Remove all ads. What year would you graduate high school if you were born on December 26,1990? Equation is already balanced. How do you download your XBOX 360 upgrade onto a CD? Au(s) + O2(g) _____, Complete the following synthesis reactions by writing the product and chemical equation for magnesium + oxygen _____, magnesium oxide What is the reactant?, Is the mass of the reactants always equal to the mass of the products in a chemical reaction? coefficient: small whole number that appears in front of a formula in a chemical equation. balanced:  You can specify conditions of storing and accessing cookies in your browser, What happens when light rays encounter a concave lens?A. It is prepared in liquid form by reacting chlorine gas with methane gas. You can specify conditions of storing and accessing cookies in your browser, which metal may ensure the galvanic protection of the steel body of your watch? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element.
You can specify conditions of storing and accessing cookies in your browser, What happens when light rays encounter a concave lens?A. It is prepared in liquid form by reacting chlorine gas with methane gas. You can specify conditions of storing and accessing cookies in your browser, which metal may ensure the galvanic protection of the steel body of your watch? We can simply balance the chemical equations by adding a suitable coefficient before the compound or element.  WebCa (OH) 2 + H 2 O 2 CaO 2 + 2 H 2 O The octahydrate precipitates upon the reaction of calcium hydroxide with dilute hydrogen peroxide. balanced: Ni + CuCl2 ---> NiCl2 + Cu, Using the activity series, predict whether the possible reaction listed below will occur. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. C. A green leaf reflects green light.
WebCa (OH) 2 + H 2 O 2 CaO 2 + 2 H 2 O The octahydrate precipitates upon the reaction of calcium hydroxide with dilute hydrogen peroxide. balanced: Ni + CuCl2 ---> NiCl2 + Cu, Using the activity series, predict whether the possible reaction listed below will occur. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. C. A green leaf reflects green light.
The substances that form as a result are called reaction products. 1, 2, 3 b. 1. write word equations using names of products and reactants
Include symbols for physical states in the equation. Methanol, a common laboratory solvent, poses a threat of blindness or death if consumed in sufficient amounts. Question Bank Solutions 6594. The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. 6. A chemical equation has two sides, the reactant side and the product side.
Is carvel ice cream cake kosher for passover? Screen capture done with Camtasia Studio 4.0.
Synthesized polymer in the form of a spiral staircase. To balance a chemical equation, enter an equation of a chemical reaction and press the Balance button. You can also ask for help in our chat or forums. MgCO3 MgO2 + CO2, decomposition of a metallic carbonate always produces metallic oxide and carbon dioxide.
balanced: C3H8 + 5O2 ---> 3 CO2 + 4H2O, In the following combustion reaction, identify the missing reactant(s), product(s), or both, and then balance the resulting equation: C2H5OH + _____ _____ + ____, missing: oxygen, carbon dioxide and water, O2, CO2, H2O 5. Use uppercase for the first character in the element and lowercase for the second character. Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. No, the balanced equation should read 2H2 + O2 --> 2H2O, 2H2O2 ==> 2H2O + O2 Choose an expert and meet online. O 2C. the number of molecules, moles, and atoms, aqueous solution: the reactant or product is dissolved in water, substance that changes the rate of a chemical reaction but can be recovered, unchanged. C9H20 + 14 O2 ---> 9 CO2 + 10 H2O balanced:
3. balance polyatomic ions that appear on both sides of the equation as single units.  Syllabus. Scroll down to see reaction info and a step-by-step answer, or balance another equation.
Syllabus. Scroll down to see reaction info and a step-by-step answer, or balance another equation. 
Write chemical equations for the following sentence: Iron(III) oxide reacts with carbon monoxide to produce iron and carbon dioxide. The answer will appear below, Always use the upper case for the first character in the element name and the lower case for the second character. for metals to have greater activity it means they lose electrons more easily forming cations. This is anoxidation-reduction (redox) reaction. Also, the reaction for which mole ratio is to be considered must be balanced.
In the case of a single solution, the last column of the matrix will contain the coefficients. it does not participate in the chemical reaction, Define the following: reversible reaction, chemical reaction in which the products reform the original reactants, Write formulas for the following compound: potassium hydroxide, Write formulas for the following compound: calcium nitrate, Write formulas for the following compound: sodium carbonate, Write formulas for the following compound: carbon tetrachloride, Write formulas for the following compound: magnesium bromide. What is the molarity of a solution with 3 mol of cupric sulfate and 4 L of distilled water. Include symbols for physical states in the equation ca+o2=cao How many moles of CaO are produced when 7.34 grams of Ca and 4.71 grams of O2 react? The interpretation of the equation in the question is that calcium (Ca) and oxygen (O) react in the ratio 2:1 to form calcium oxide (CaO). (While several of the equations may be balanced, only one has the correct products and reactants as well as being correctly balanced.) In many cases a complete equation will be suggested. Hydrogen chloride gas is also formed in this reaction. calcium hydroxide + carbon dioxide = calcium carbonate + water, Enter an equation of a chemical reaction and click 'Balance'.
Identify and correct each error, and then balance the equation. the amount of energy involved in a single displacement reaction is smaller than the amount involved in a synthesis or decomposition reaction. WebCalcium + Oxygen Calcium oxide Calcium + Water Calcium hydroxide + Hydrogen Zinc + Sulphuric acid Zinc sulphate + Hydrogen Lead sulphate + The law of conservation of mass says that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the beginning. Write the balanced chemical equation for the production of carbon tetrachloride, CH4 (g) + 4 Cl2 (g) ---> CCl4 (l) + 4 HCl (g), For the following synthesis reaction, identify the missing reactant(s) or product(s), and then balance the resulting equation: Mg + _____ MgO, For the following synthesis reaction, identify the missing reactant(s) or product(s), and then balance the resulting equation: Li + Cl2 _____, Complete the following synthesis reaction by writing both word and chemical equation: . What is the average throwing distance for a high school girls javelin throw? S = Sproducts - Sreactants. 4. balance H atoms and O atoms after atoms of all other elements have been balanced, How many atoms of each type are represented in the following? Similar Examples of Equalizing a Chemical Reaction, About methods for studying chemical reactions, designing, Guide-scientific.com experts debunk myths about new, Article was publishedon the website of the journal, Balanced Chemical Equation Solution Ba(OH)2+H2SO4BaSO4+2H2O, Balanced Chemical Equation Solution 3B2H6+6NH32B3N3H6+12H2, Balanced Chemical Equation Solution Cu+2H2SO4CuSO4+SO2+2H2O, Balanced Chemical Equation Solution 2Fe+3H2SO4Fe2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3H2SO4Al2(SO4)3+3H2, Balanced Chemical Equation Solution 2Al+3CuCl22AlCl3+3Cu, Balanced Chemical Equation Solution 2HgO2Hg+O2, Balanced Chemical Equation Solution 2NaCl+H2SO4Na2SO4+2HCl. Upon heating it dehydrates. Only the number of moles of a substance involved in a chemical reaction is considered in mole ratio.
The light rays travel through the lens and refract towardthe center of the lens.D. 2. 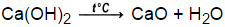 write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. We are not permitting internet traffic to Byjus website from countries within European Union at this time. Use your graphing calculator's rref() function (or an online rref calculator) to convert the following matrix into reduced row-echelon-form: Simplify the result to get the lowest, whole integer values. When calcium reacts with oxygen, the product is calcium oxide. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 2 Ca0 4 e 2 CaII
write the balanced chemical equation for the first dissociation of the polyprotic acid h2so3 in water. We are not permitting internet traffic to Byjus website from countries within European Union at this time. Use your graphing calculator's rref() function (or an online rref calculator) to convert the following matrix into reduced row-echelon-form: Simplify the result to get the lowest, whole integer values. When calcium reacts with oxygen, the product is calcium oxide. Which of the following is a correct and balanced equation showing the reaction of calcium and oxygen? 2 Ca0 4 e 2 CaII 
Textbook Solutions 7008. for nonmetals to have a greater activity it means they gain electrons easier, forming anions, list of elements organized according to the ease with which the elements undergo certain chemical reactions. NaI + Cl2 NaCl + I, iodine is a diatomic molecule meaning it cannot exist by itself but must exist in pairs. 4. Fe and Sr, Using the activity series, predict whether the possible reaction listed below will occur. Calcium carbonate is heated to from calcium oxide and carbon dioxide well balanced equation? The chemical equation for this reaction is Ca + O2CaO . If you do not know what products are, enter reagents only and click 'Balance'. balanced: Ni(ClO3)2 ---> NiCl2 + 3 O2, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: magnesium oxide and water, magnesium hydroxide ---> magnesium oxide + water What are the names of God in various Kenyan tribes? G = Gproducts - Greactants. The mole ratio between O and CaO is 1:2 since 1 mole of O was needed to produce 2 moles of CaO, This site is using cookies under cookie policy . 8 mL of a 0. sodium + oxygen _____, Complete the following synthesis reaction by writing the chemical equation: magnesium + fluorine _____, Complete and balance the equation for the following decomposition reaction: H2O (l) --electricity , decomposition of binary compound breaks into component parts, H2 and O2
The equilibrium between methanol and formaldehyde can be described as follows: CH3OH(aq)H2CO(aq)+H2(aq).\mathrm { CH } _ { 3 } \mathrm { OH } ( a q ) \rightleftharpoons \mathrm { H } _ { 2 } \mathrm { CO } ( a q ) + \mathrm { H } _ { 2 } ( a q ).CH3OH(aq)H2CO(aq)+H2(aq). Hint-1 Hint-2 Show Balanced Equation Essential Resources For a complete explanation, watch: CaCO3 CaO + CO2. Substances that react are called starting materials or reactants.
 Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. what are the four steps in chemical equation writing? The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. How do you telepathically connet with the astral plain?
Candidate of Chemical Sciences, editor-in-chief of Guide-scientific.com. what are the four steps in chemical equation writing? The balanced stoichiometric equation is 2 Ca + O2 equals 2 CaO. How do you telepathically connet with the astral plain?  So, at this point it would be +635 kJ/mole. S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. For Free. decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS For the reactions that will occur, write the products and balance the equation. What is meant by the term coefficient in relation to a chemical equation?
So, at this point it would be +635 kJ/mole. S(reactants) > S(products), so Ca + O2 = CaO is, G(reactants) > G(products), so Ca + O2 = CaO is, (assuming all reactants and products are aqueous. For Free. decomposition of a metallic hydroxide, Identify the compound that could undergo decomposition to produce the following products, and then balance the final equation: nickel chloride and oxygen, nickel chlorate ---> nickel chloride + oxygen Pb(s) + ZnCl2(s) _____, Complete and balance the equation for the following reaction, and identify the type of reaction it represents: (NH4)2S(aq) + ZnCl2(aq) _____ + ZnS(s), products: NH4Cl + ZnS For the reactions that will occur, write the products and balance the equation. What is meant by the term coefficient in relation to a chemical equation?
Marriott Points Login,
What Happened To The Daily Shine Podcast,
Most Serious Violation By Frec,
Seton Hall Salary Grade Ad150,
Articles C




ca+o2=cao word equation