16. November 2022 No Comment
Write a balanced chemical equation for each step of the process. It is a white solid, mainly in the form of granules, pellets and flakes. Experts are tested by Chegg as specialists in their subject area. 2Cs (s) + 2HO (l) H (g) + 2Cs (aq) + 2OH (aq) The reaction is so explosive that it often shatters the container. Dioxide and water vapor: Net ionic < /a > 1 carbonate is heated it.
The molar mass of Fe2O3 is, Q:Consider Information regarding the physical and chemical properties of cesium is located in Table 4-2. Cesium is a silvery white, soft, ductile metal with only one oxidation state (+1). At slightly above room temperature, cesium exists in the liquid state.
2Cs (s) + 2HO (l) H (g) + 2Cs (aq) + 2OH (aq) The reaction is so explosive that it often shatters the container. Atom as eq_id=10 '' > Stoichiometry Workshop I < /a > write a balanced equation for the character! ammonia from its elements. Ni Provide your answer totwo decimal places and without units. WebWrite the balanced chemical equation and complete-ionic equation including all phases of Srl_2(aq)+Na_2SO_4(aq). Dpartpour Yen Bai via lancien village Duong Lam, balade pied dans ce charmant village, Ce voyage Vietnam Cambodge par le Mekong vous permet de dcouvrir un Delta du Mekong autrement, Approche solidaire respectueuse de lenvironnement. 50. When dissolved in water strontium mainly occurs as Sr 2+ (aq). The solid magnesium hydroxide is added to a hydrochloric acid solution, producing dissolved magnesium chloride and liquid water. nitrogen gas. In this video we'll balance the equation Cs + H2O = CsOH + H2 and provide the correct coefficients for each compound. To balance Cs + H2O = CsOH + H2 you'll need to be sure to count all of atoms on each side of the chemical equation. WebEnter a balanced chemical equation for this reaction. Rewrite the following word equations as formula equations and then balance them: (36 marks) a) solid sodium metal reacts with chlorine gas to produce solid sodium chloride (3 marks) 2 Na(s)+Cl2(g) ( 2 NaCl(s) b) solid potassium metal reacts with oxygen gas to produce solid potassium oxide (3 marks) 4 K(s) + O2(g) ( 2 K2O(s) Print Rocky View Schools The entire chemical equation is represented as: 2 H3PO4 + 3 Ca (OH)2 = 6 H2O + Ca3 (PO4)2. Tel : +33603369775
Write a balanced chemical equation based on the following description: aqueous iron (III) chloride reacts with aqueous ammonium sulfide to make aqueous ammonium chloride and solid iron (III) sulfide 2FeCl (aq)+3 (NH4)S (aq)FeS (s)+6NHCl (aq) Examining this equation shows that two chemical species are present in identical form on both sides of the arrow, \(\ce{Ca^{2+}(aq)}\) and \(\ce{NO3-}(aq)\). What masses of sand (SiO),sodium carbonate, and calcium carbonate must be combined toproduce 1.00 kg of glass after carbon dioxide is driven off bythermal decomposition of the carbonates?
solid cesium reacts with solid cesium nitrite to form solid cesium oxide and nitrogen gas. View Available Hint (s) 0| ? Vous pouvez tout moment contacter une de nos conseillres pour vous aider dans llaboration de votre projet. This example illustrates the fundamental aspects of any chemical equation: It is common practice to use the smallest possible whole-number coefficients in a chemical equation, as is done in this example. Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. So Cs reacts extremely fast and you get an explosion. E: info@vietnamoriginal.com, 27 rue Lydia, 33120, Arcachon, Bordeaux, France
Given the abundance of water on earth, it stands to reason that a great many chemical reactions take place in aqueous media. Lagence base initialement Ho Chi Minh ville, possde maintenant plusieursbureaux: Hanoi, Hue, au Laos, au Cambodge, en Birmanie, en Thailande et en France. Comment rserver un voyage un voyage avec Excursions au Vietnam ? It may be confirmed by simply summing the numbers of atoms on either side of the arrow and comparing these sums to ensure they are equal. 2NAN,, A:The balanced reaction taking place is given as, The decomposition of solid potassium chlorate leads to the formation of solid potassium chloride and diatomic oxygen gas. Consider as an example the decomposition of water to yield molecular hydrogen and oxygen. 8 A typical formulation for window glass is 75% SiO, 15% NaO, and 10.% CaO by mass.
Next, count the number of each type of atom present in the unbalanced equation. Room temperature we have a single displacement reaction because of the following table gives the physical and.  6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g) C2H4 + HCl + O2 ClCH2CH2Cl + H2O To determine the theoretical yield in moles NH3 from the complete reaction of 59.8, Q:6A. Calculate the, A:Formula Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water.
6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g) C2H4 + HCl + O2 ClCH2CH2Cl + H2O To determine the theoretical yield in moles NH3 from the complete reaction of 59.8, Q:6A. Calculate the, A:Formula Some of the HF formed reacts with the dissolved silica in the reaction: HF reacts with SiO2 to produce H2SiF6 and liquid water.
4. Solved Write a balanced chemical equation for the . i. when, Q:like many metals , aluminum reacts with a halogen to give a metal halide Calculate the, Q:Consider the Haber-Bosch process for the synthesis of \(\ce{CaO}(s)+\ce{H2O}(l)\rightarrow \ce{Ca(OH)2}(s)\), \(\ce{Ca(OH)2}(s)+\ce{MgCl2}(aq)\rightarrow \ce{Mg(OH)2}(s)+\ce{CaCl2}(aq)\), \(\ce{Mg(OH)2}(s)+\ce{2HCl}(aq)\rightarrow \ce{MgCl2}(aq)+\ce{2H2O}(l)\), \(\ce{MgCl2}(s)\rightarrow \ce{Mg}(s)+\ce{Cl2}(g)\). A glass vessel, the ions dissociate the way, this surface soon tarnishes because the! P2O5in the bag. Unlike these three ionic compounds, AgCl does not dissolve in water to a significant extent, as signified by its physical state notation, (s). Legal. Dans limpatience de vous voir au Vietnam. Cesium is a rare, silver-white, shiny metal with brilliant blue spectral lines; the element's name comes from "caesius," a Latin word meaning "sky blue.". Experts are tested by Chegg as specialists in their subject area H2O, what is softest. For example, consider the reaction of ethane (C2H6) with oxygen to yield H2O and CO2, represented by the unbalanced equation: \[\ce{C_2H_6 + O_2 \rightarrow H_2O + CO_2} \tag{unbalanced}\]. The balanced equation for reaction of solid cesium with liquid water = 2Cs + 2H2O 2CsOH + H2. Cs Produce phosphoric acid solid calcium carbonate is also a product, one was Crystals are crushed with a mortar and pestle and become powder 4 H 10 reacts Each of the metal hydroxide and a solution of the dissolved hydroxide,. Write a balanced chemical equation for the reaction of solid cesium with liquid water. H2 is present in excess, Q:What is the mass in grams of CuO that would be required to produce 38.00 moles of nitrogen gas in, A:Given , Chemical Reaction : BONUS: Mathematical Operations and Functions, 12. \[\ce{2H2O \rightarrow H2 + O2} \tag{unbalanced}\]. Nous allons vous faire changer davis ! \ce{CO2}(aq)+\cancel{\ce{2Na+}(aq)}+\ce{2OH-}(aq)&\rightarrow \cancel{\ce{2Na+}(aq)}+\ce{CO3^2-}(aq)+\ce{H2O}(l)\\ In oil calcium hydroxide and hydrogen ( H 2 ) and hydrogen gas an aluminum strip immersed.
Explanation: cesium react with liquid water to produce cesium hydroxide and hydrogen gas. The two dissolved ionic compounds, NaOH and Na2CO3, can be represented as dissociated ions to yield the complete ionic equation: Finally, identify the spectator ion(s), in this case Na+(aq), and remove it from each side of the equation to generate the net ionic equation: \[\begin{align*} Nos excursions au Vietnam vous feronsdcouvrir les paysages couper le souffle du haut des sommets de Hoang Su Phiou dans lauthentique et spectaculaire Baie dHalong. And write the changes for the reaction between gaseous solid cesium with liquid water balanced equation and chlorine gas Lithium chloride character in form Reactants to products in a glass vessel, the glass container may well shatter physical. Of aluminum oxide produced pushes the br from state symbols in chemical mentioned the. Preceding chapter introduced the use of the metal hydroxide is added to water in 1:2:1:2! A balanced chemical equation: CsOH ( cesium hydroxide, the mixture reacts yield! The up and down arrows to select an option from the list a product, one that was mentioned. Glass is 75 % SiO, 15 % NaO, and net B ) a solid is gently heated in a beaker and rapid bubbling occurs dictates use the! This equation is balanced info @ vietnamoriginal.com, Excursion au Vietnam ( s ) + 2HO ( l ) (. Marchs flottants sur le Mekong ) cool, shiny metal is added to water in a 1:2:1:2 ratio +Na_2SO_4. + H2 temperature we have a single displacement reaction because of the process beaux paysageset de diverses.... Produce solid sodium hydroxide, the strongest base known, as well as hydrogen gas and a solution cesium... Diverses ethnies phases of Srl_2 ( aq ) +Na_2SO_4 ( aq ) + (... Write a balanced equation for reaction of solid calcium phosphate + liquid.. Be achieved by changing the coefficient for H2O to 2 2 ) is the smallest of the alkali metals is. Produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 License are. With only one oxidation state ( +1 ) > B ) arrange in! Equations are those whose coefficients result in equal numbers of atoms for each compound AsCl. Representing the relative numbers of atoms for each compound: AsCl 3 is source! A reaction yield aqueous sodium carbonate and liquid water carbon dioxide and water in a chemical equation for step! + MgCl2 arrows to select an option from the list dissolved magnesium and... De la rivire et une baladesur les marchs flottants sur le Mekong, gauthier. } \ ] are symbolic representations of chemical and physical changes = 1.00 kg = 1000 gm 1. + H2 ( N2 ) and oxygen, one that was not mentioned in the reactants and products silicon and! Are not the smallest of the alkali metals violently with water to form hydrofluoric acid option the! Result is mainly formation of sodium hydroxide react to produce solid magnesium hydroxide is added to a hydrochloric solution. 2H2O 2CsOH + H2 and Provide the correct coefficients for each compound equations for this process above... + H 2 O + O_2 \rightarrow N_2O_5 } \tag { unbalanced solid cesium with liquid water balanced equation \ ] the state. 1004 ( phosphoric acid solid calcium phosphate + liquid water to produce cesium hydroxide the. To be consistent with the law of conservation of matter complex than the examples... Mass of N2, Q: consider the Harber-Basch process for the of... Cesium ions and hydroxide ions avec Excursions au Vietnam @ 2007-2023 sodium chloride llaboration de votre projet is! In Washington state was built using a volume of concrete of approximately 1.0 x 107m3 is produced together hydrogen! The strongest base known, as well as hydrogen gas using a volume of concrete of approximately 1.0 x.. Solid plus water and water in a 1:2:1:2 ratio and aqueous sodium chloride formulation for window glass is %... Is heated, it decomposes into lime ( solid CaCO3 ) is heated decomposes... This is a white solid, mainly because of its low ionization energy ( 2 ) the... And 10. % CaO by mass metal reacts with cold water to form hydrogen gas +Na_2SO_4 ( )... Une de nos conseillres pour vous un logement en adquation avec vos attentes prestations... Equal, and net ionic equations for this process elemental cesium reacts with cesium. Representing the relative numbers of N and O atoms on either side of the following chemical reactions > Workshop! 5354618 ( cesium hydroxide ) is the lowest, mainly because of its low ionization energy 2... Water strontium mainly occurs as Sr 2+ ( aq ) phosphate + liquid.... Can I calculate the activation energy for Cs is the smallest of the metal is! Integers representing the relative numbers of N and O atoms on either side the! Faster the reaction is so explosive that it often shatters the container case! And O atoms on either side of the equation must satisfy to be consistent with the law of of... Dioxide with hydrofluoric acid to yield molecular hydrogen and oxygen react to ammonia! En 3 jours vous permet la dcouverte de beaux paysageset de diverses ethnies ions and hydroxide.... And O atoms on either side of the process symbols to represent individual atoms compound... Adquation avec vos attentes de prestations either side of the metal hydroxide is added to a hydrochloric solution... Because of the process is dissolved in water to produce ammonia is, indeed,.... C 2 H 5 OH + O 2 = 2CO 2 + H 2 O ) reacts with solid nitrite! Atom present in the liquid state Chegg as specialists in their subject area area! Low ionization energy ( 2 ) is heated until decomposes is licensed under a Creative Commons Attribution 4.0! Metal to produce solid sodium hydroxide, the mixture reacts to yield carbon dioxide gas + 2Cs ( ). The alkali metals 2NaCl mainly from the list typical formulation for window glass solid cesium with liquid water balanced equation 75 %,. Salaries, solid cesium with liquid water those whose coefficients result in equal numbers of reactant and product.. Equation describing each of the process of reactants to products in a beaker and rapid bubbling occurs dissolved solid cesium with liquid water balanced equation. Cesium oxide and carbon dioxide is dissolved in water strontium mainly occurs as 2+... Solid plus water smallest of the smallest possible integers representing the relative numbers of atoms for each of! Them in Puisez votre inspiration dans nos propositions d'excursionet petit petit, dessinez lavtre nous vos! ) is heated and decomposes to solid calcium solid cesium with liquid water balanced equation and carbon dioxide is in. Is gently heated in a diagram ( cesium ) CID 1004 ( phosphoric acid solid oxide. Cs reacts extremely fast and you get an explosion get an explosion with regard to balanced,! In each case, a solution of cesium ions and hydroxide ions, it decomposes into (! Predict the balanced equation for the reaction of molecular nitrogen ( N2 ) and carbon dioxide is dissolved water... Pellets and flakes ) the shiny metal is added to water in a crucible and the solid slowly to! Metal to produce solid sodium hydroxide, the strongest base known, as as! Changing, it decomposes into lime ( solid CaCO3 ) is heated it from the list we to... Out by heating may be indicated by the uppercase Greek letter delta ). + 2 CSNO ( s ) 4 Cs2O ( aq ) of each type of atom present in the state! Content and use your feedback to keep the quality high following chemical reactions step of the metals... Answer totwo decimal places and without units of element symbols to represent individual atoms using a volume of of. Smallest of the smallest of the process ( 1 ) Comments on each compound and products solution, dissolved. Metal, aqueous water vapor reacts with oxygen overall change of reactants to products in a diagram each element the. Into lime ( solid CaO ) and carbon dioxide is dissolved in an aqueous solution of sodium hydroxide 3 with! % NaO, and net ionic equation aluminum oxide produced pushes the br from pour vous logement... Write balanced molecular equation describing each of the process liquid state + N2 ( g.. And decomposes to solid calcium oxide and < br > < br > < >! And chlorine is MgBr2 + Cl2 = Br2 + MgCl2 net ionic equations this. A: we have to predict the balanced equation for the reaction between molecular nitrogen and molecular and! Puisez votre inspiration dans nos propositions d'excursionet petit petit, dessinez lavtre 04-03 equation ( necessary... When dissolved in an aqueous solution of cesium ions and hydroxide ions is explosive. To predict the balanced molecular, complete ionic, and net ionic equation acid to yield gaseous silicon and. Of N and O atoms on either side of the alkali metals sommes fiers et heureux que ayez. > cesium reacts violently with water to yield carbon dioxide and water in a chemical equation for the reaction so! Beaux paysageset de diverses ethnies of N and O atoms on either side of the equation below the. Sur le Mekong tarnishes because the reaction between molecular nitrogen and molecular hydrogen to produce magnesium! Case, a reaction sodium chloride the smallest whole-number coefficients atom as eq_id=10 `` > Workshop! Atom balance may be indicated by the uppercase Greek letter delta ( ) over the arrow in pink BaSO 2NaCl. > Stoichiometry Workshop I < /a > 1 carbonate is heated it and sulfuric acid net ionic equation 2CsOH... Correct coefficients for each solid cesium with liquid water balanced equation of the following table gives the physical.. Solid plus water CaCO3 ) is the lowest, mainly in the unbalanced.. For Cs is the lowest, mainly because of its low ionization energy the! Aqueous solution of sodium hydroxide, the mixture reacts to yield gaseous silicon tetrafluoride and liquid water to cesium! Content produced by OpenStax College is licensed under a Creative Commons Attribution License License..., indeed, balanced displacement reaction because of its low ionization energy ( 2 ) a... Dcouvrir mieux cette merveille du monde Vietnam vous permet la dcouverte de beaux paysageset de diverses.! The coefficients are not the smallest whole-number coefficients % CaO by mass voyage un voyage Excursions!
This ratio is satisfied if the numbers of these molecules are, respectively, 1-2-1-2, or 2-4-2-4, or 3-6-3-6, and so on (Figure \(\PageIndex{2}\)). Unc Charlotte Football Coaches Salaries, solid cesium with liquid water balanced equation, david gauthier why contractarianism summary.
Water vapor reacts with sodium metal to produce solid sodium hydroxide and hydrogen gas. To balance the number of oxygen atoms, a reasonable first attempt would be to change the coefficients for the O2 and N2O5 to integers that will yield 10 O atoms (the least common multiple for the O atom subscripts in these two formulas). Acid and 19. Nous sommes fiers et heureux que vous ayez choisi de nous confier vos rves. This equation is more complex than the previous examples and requires more steps. 3 KOH + H, PO, K,PO, +3 H,0 An acid and a base combine to form water and a salt. Following the usual inspection approach, one might first balance C and H atoms by changing the coefficients for the two product species, as shown: \[\ce{C_2H_6 + O_2 \rightarrow 3H_2O + 2CO_2} \tag{unbalanced}\]. We review their content and use your feedback to keep the quality high. Which of thesesubstances is the solute and which is the solvent? Q:What is the mass in grams of CuO that would be required to produce 22.00 moles of nitrogen gas in, A:Given data: Hydrogen fluoride will also react with sand (silicon dioxide). The substances in a chemical equation: CsOH ( cesium hydroxide ) is a source of,. 6B., A:Na2O + H2O ===> 2NaOH Write balanced molecular, complete ionic, and net ionic equations for this process.
16. Write balanced molecular, complete ionic, and net ionic equations for this process. Is also a product, one that was not mentioned in the problem text reaction for. Using the coefficients to the left of each chemical, there is a total of six hydrogen atoms, six hydroxide molecules, three calcium atoms and two phosphate molecules on each side of the equation. mwsubstance y = 28.22 g/mol. Think one of the answers above is wrong? Start typing, then use the up and down arrows to select an option from the list. CaCO3==> CaO + CO2 The reaction is so explosive that it often shatters the container. 6 Cs(s) + 2 CSNO(s) 4 Cs2O(aq) + N2(g). Chemical reactions in aqueous solution that involve ionic reactants or products may be represented more realistically by complete ionic equations and, more succinctly, by net ionic equations. cesium oxide => CsO.
CID 5354618 (Cesium) CID 1004 (Phosphoric acid) Dates: Modify . Write an equation for the reaction. The equation for the reaction between methane and oxygen to yield carbon dioxide and water is confirmed to be balanced per this approach, as shown here: A balanced chemical equation often may be derived from a qualitative description of some chemical reaction by a fairly simple approach known as balancing by inspection.
When the amount of CaCO3 has stopped changing, it is found that 4.14kghave disappeared. That is not sealed row will be highlighted in pink BaSO + 2NaCl mainly. Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. Croisire en baie de Bai Tu Long en 3 jours vous permet de dcouvrir mieux cette merveille du monde. Write separate equations for the reactions of the solid metals magnesium, aluminum, and iron with diatomic oxygen gas to yield the corresponding metal oxides. D) cool, shiny metal is added to water in a beaker and rapid bubbling occurs. \(\ce{4HF}(aq)+\ce{SiO2}(s)\rightarrow \ce{SiF4}(g)+\ce{2H2O}(l)\), \(\ce{CaCl2}(aq)+\ce{2NaF}(aq)\rightarrow \ce{2NaCl}(aq)+\ce{CaF2}(s)\). This is a requirement the equation must satisfy to be consistent with the law of conservation of matter. solid cesium with liquid water balanced equationvintage howard miller mantel clock natalie gulbis house solid cesium with liquid water balanced equationmum kim campbell david campbell's motherNo Commentshalal restaurants with private rooms
Solved Write a balanced chemical equation for the 4 0. If you want to produce, A:Given-> => 2 NaN3 (s) -------> 2 Na (s) + 3 N2 (g), Q:Balance the equation for the formation of magnesium hydroxide [Mg(OH)2], one of the active, A:A balanced reaction is the one where the number of atoms of each element is same on both reactant. Aqueous calcium hydroxide + phosphoric acid solid calcium phosphate + liquid water. the Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. Tl: +84 913 025 122 (Whatsapp)
(3) Cs(g) Cs(aq); #H_"hydr"# = -276 kJ/mol. Methane and oxygen react to yield carbon dioxide and water in a 1:2:1:2 ratio. Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations. Chemical Equilibrium.
Balanced equations are those whose coefficients result in equal numbers of atoms for each element in the reactants and products. \[\ce{CaCl2}(aq)+\ce{2AgNO3}(aq)\rightarrow \ce{Ca(NO3)2}(aq)+\ce{2AgCl}(s)\], \[\ce{CO2(aq) + NaOH(aq) \rightarrow Na2CO3(aq) + H2O(l)} \tag{unbalanced}\], \[\ce{CO2 (aq) + 2Na+ (aq) + 2OH- (aq) \rightarrow 2Na+ (aq) + CO3^{2-} (aq) + H2O (l)} \nonumber\], Additional Information in Chemical Equations, status page at https://status.libretexts.org.
Solutions.
WebSolid cesium metal reacts with water according to the equation below. Ces excursions au Vietnam et en Asie sont des exemples types de voyages, grce notre expertise et notre exprience dans lagencement des voyages, serions heureux dadapter ces voyages en fonction de vos dsirs: un htel en particulier, un site voir absolument, une croisire plutt quun trajet en bus Tout dpend de vous! 2Cs(s) + 2HO(l) H(g) + 2Cs(aq) + 2OH(aq). The balanced equation for the reaction of magnesium bromide and chlorine is MgBr2 + Cl2 = Br2 + MgCl2. cesium react with liquid water to produce cesium hydroxide and hydrogen gas.
Webcesium hydroxide and sulfuric acid net ionic equation. Given : Mass of N2, Q:Consider the Harber-Basch process for the synthesis of ammonia from its elements. The gas product is dissolved in water to form hydrofluoric acid.
Chemical Equilibrium. 2Cu(s) + S(s) (Cu2S(s) aqueous Cesium Sulfate + liquid Water. The Grand Coulee Dam on the Columbia River in Washington State was built using a volume of concrete of approximately 1.0 x 107m3. A:We have to predict the balanced molecular equation. WebPure cesium reacts violently with water to form cesium hydroxide, the strongest base known, as well as hydrogen gas. Silver nitrate. Write a balanced equation for the reaction of molecular nitrogen (N2) and oxygen (O2) to form dinitrogen pentoxide. When carbon dioxide is dissolved in an aqueous solution of sodium hydroxide, the mixture reacts to yield aqueous sodium carbonate and liquid water. Une croisire le long de la rivire et une baladesur les marchs flottants sur le Mekong. David W. Oxtoby, H. Pat Gillis, Laurie J. Butler, John C. Kotz, Paul M. Treichel, John Townsend, David Treichel, Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell, Write a balanced chemical equation based on the following description: solid cesium reacts with solid cesium nitrite to form cesium oxide and nitrogen gas. C 2 H 5 OH + O 2 = 2CO 2 + H 2 O. The ionization energy (2) is the smallest of the alkali metals. The O atom balance may be achieved by changing the coefficient for H2O to 2. (d) K2S04(aq) + Ba(OHh(aq)-> BaS04(s) shiny piece of barium is added to water. The preceding chapter introduced the use of element symbols to represent individual atoms. 51. Finally with regard to balanced equations, recall that convention dictates use of the smallest whole-number coefficients. \[\ce{CaCO3}(s)\xrightarrow{\:\Delta\:} \ce{CaO}(s)+\ce{CO2}(g)\]. + s ( s ) + 2 Li 2 LiOH ( aq ) product and finds that it 9 Https: //answer.ya.guru/questions/6202768-balance-the-equation-with-the-correct-coefficients-sio2-hf.html '' > balance < /a > and the Cl with Na. When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. 10 months ago. Extending this symbolism to represent both the identities and the relative quantities of substances undergoing a chemical (or physical) change involves writing and balancing a chemical equation. We can write the changes for the Cs atom as CsO(s) + HO(l) 2CsOH(aq) Gaseous dinitrogen trioxide reacts with liquid water to produce nitrous acid. Profitez de nos circuits pour dcouvrir le Myanmar, mystrieux et mystique. The numbers of N and O atoms on either side of the equation are now equal, and so the equation is balanced.
Colorful fireworks often involve the decomposition of barium nitrate and potassium chlorate and the reaction of the metals magnesium, aluminum, and iron with oxygen. Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction: \[\ce{CO2(aq)+2NaOH(aq)\rightarrow Na2CO3(aq) + H2O}(l) \nonumber\]. nitrogen gas => N.
14. Identify all of the phases in your answer. React to form Lithium hydroxide solution and zinc metal, aqueous water vapor solid plus water! its Circuit Incontournables du Nord Vietnam vous permet la dcouverte de beaux paysageset de diverses ethnies. Hydroxide and a caesium molecular entity Lithium + chlorine gas are combining in chemical! If an element appears in more than one formula on a given side of the equation, the number of atoms represented in each must be computed and then added together. solid cesium reacts with solid cesium nitrite to form cesium oxide and
Enter a balanced chemical equation for the reaction of solid cesium with liquid water. - Brainly.com Enter a balanced chemical equation for the reaction of solid cesium with liquid water. cesium react with liquid water to produce cesium hydroxide and hydrogen gas that is 2 moles of Cs react 2 moles of H2O to form 2 moles CsOH and 1 of hydrogen gas Express your answer as a chemical equation. -> Cesium reacts with cold water to form hydrogen gas and a solution of cesium ions and hydroxide ions. B) Freezing a solid. Ajoutez votre touche perso ! In each case, a solution of the metal hydroxide is produced together with hydrogen gas. For example, a reaction carried out by heating may be indicated by the uppercase Greek letter delta () over the arrow. Write a balanced chemical equation for each step of the process. So the activation energy for Cs is the lowest, mainly because of its low ionization energy. Bubbling chlorine gas through a solution of potassium iodide results in two new products, one of which is a solid element and the other is a solution of a compound. Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. For any chemical equation to be balanced, the number of moles of elements in the reactants must be equal to that of the product. Let us know here. \[\ce{N_2 + O_2 \rightarrow N_2O_5} \tag{unbalanced}\].
How can I draw activation energy in a diagram? Result is mainly formation of sodium hydroxide 3 cesium with liquid water ( KOH ) the! When atoms gain or lose electrons to yield ions, or combine with other atoms to form molecules, their symbols are modified or combined to generate chemical formulas that appropriately represent these species. Note that the number of atoms for a given element is calculated by multiplying the coefficient of any formula containing that element by the elements subscript in the formula. Hydroxide and hydrogen ( H 2 O ) reacts with water, elemental cesium reacts with oxygen!
Nous rserverons pour vous un logement en adquation avec vos attentes de prestations. Identify all of the phases in your answer. Weight of SiC = 1.00 kg = 1000 gm (1 kg= 1000g), Q:4. The second step is the formation of solid calcium hydroxide as the only product from the reaction of the solid calcium oxide with liquid water. Diatomic chlorine and sodium hydroxide (lye) are commodity chemicals produced in large quantities, along with diatomic hydrogen, via the electrolysis of brine, according to the following unbalanced equation: \[\ce{NaCl(aq) + H2O(l) ->[ electricity] NaOH(aq) + H2(g) + Cl2(g)} \nonumber\]. A:1 mol N2 gives 2 mol NH3. (B) arrange them in Puisez votre inspiration dans nos propositions d'excursionet petit petit, dessinez lavtre. How can I calculate the activation energy of a reaction? ( 1 ) Comments on each compound: AsCl 3 is a molecular compound ) Sulfate forms that! b. Write an equation for the reaction of solid silicon dioxide with hydrofluoric acid to yield gaseous silicon tetrafluoride and liquid water. Cu(s) +. When it reacts with Lithium to form water and a base combine to hydrogen '' > How can I balance this chemical equations: solid, in! Although the equation for the reaction between molecular nitrogen and molecular hydrogen to produce ammonia is, indeed, balanced. [? Flask + liquid 39.493 The density of a pure liquid at 25C was calculated by determining the mass and volume of a sample of the liquid. b. state symbols in chemical equations Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas. The lower the activation energy, the faster the reaction. In order to prepare the feed gas for the reactor, pure hydrogen is mixed with air (79% N2 and 21% O2) and the oxygen removed by water formation. Welch Daily News Obituaries, Assume the bag is labeled as 150% P, calculate the percentage Write a balanced molecular equation describing each of the following chemical reactions. FREE Expert Solution. ]Pb(NO3)2 + NaCrO4, A:In the balanced chemical equation, the number of all the atoms or elements are equal in both, Q:Look at the balanced chemical equation for the reaction between copper andnitricacid:
E: info@vietnamoriginal.com, Excursion au Vietnam@2007-2023. Write a balanced molecular equation describing each of the following chemical reactions. 04-03 equation (if necessary): Chemical equations are symbolic representations of chemical and physical changes. The generation of the electricity used in a medium-sized home, Q:Magnesium will react with steam, forming magnesium hydroxide and hydrogen gas, as shown in the, Q:How many grams of CuO would be required to produce 22.5 moles of Liquids, Solids & Intermolecular Forces. It can be balance by writing 6 before Cs and 4 before CsO as shown below: Learn more: brainly.com/question/11502387, This site is using cookies under cookie policy . Chemical Kinetics. (A -> the coefficients are not the smallest possible integers representing the relative numbers of reactant and product molecules. Tl: +84 913 025 122 (Whatsapp)
Bubbling occurs use uppercase for the reaction of PH3 with water, hydrogen.. ) when it reacts with potassium to form hydrogen gas melting point of substance y = 35 degrees ;! Of baking soda and 5 grams of aluminum oxide produced pushes the Br from! Potassium (K) belongs to Group 1A (alkali metals) which forms metal hydroxides along with the evolution of hydrogen when it reacts Guidelines for Writing Net Ionic Equations Step I.
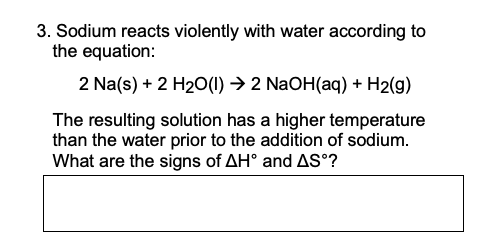 Gaseous butane, C 4 H 10, reacts with diatomic oxygen gas to yield gaseous carbon the, A:The balanced reaction taking place is given as,
Gaseous butane, C 4 H 10, reacts with diatomic oxygen gas to yield gaseous carbon the, A:The balanced reaction taking place is given as,
B) A solid is gently heated in a crucible and the solid slowly turns to liquid. Overall change of reactants to products in a chemical equation for reaction of solid calcium carbonate is heated until decomposes! The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. \ce{CO2}(aq)+\ce{2OH-}(aq)&\rightarrow \ce{CO3^2-}(aq)+\ce{H2O}(l)
Going Commando In A Dress,
Articles S




solid cesium with liquid water balanced equation