16. November 2022 No Comment
C5H8 Which of the following formed? CH3 0000007129 00000 n
Retinol How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? Metathesis Reactions: Sodium carbonate + sulfuric acid Acids
While the clever hack cleaned the grout effectively and the Aussie mum was pleased the results, it's worth noting for health and safety reasons that it's advised to avoid mixing bleach with other chemicals. Which of the following reactions are metathesis reactions?
*Response times may vary by subject and question complexity. What is his mass in kilograms? Citric acid is often found in soft drinks. C40 H78 Mix some toilet bowl cleaner with bleach in a container and leave the concoction in the house overnight.
0000014891 00000 n
What is the molecular equation for this reaction? Metathesis Reactions: Copper(II) sulfate + sodium phosphate Write a balanced chemical equation showing how you could prepare the following salt from an acid-base reaction: NaBr, Write a balanced chemical equation showing how you could prepare the following salt from an acid-base reaction: CaS. Metathesis Reactions: Nickel chloride + silver nitrate Give two balanced chemical equations showing how a salt is formed when an acid reacts with a base. While a mixture with bicarb is considered safe, some group members used the post as an opportunity to flag the very real dangers of combining potent chemicals in cleaning products. WebLecture note experiment 13 volumetric analysis ii: determination of active ingredients in commercial bleach and vinegar outcomes after completing this Skip to document
Besides chlorine gas after mixing these two chemicals what liquid byproduct is left? Metathesis Reactions: Nickel chloride + sodium carbonate Metathesis Reactions: Cadmium chloride + sodium sulfide What is his height in centimeters? Mixing bleach and ammonia can be deadly. When combined, these two common household cleaners release toxic chloramine gas. Exposure to chloramine ga "Good on you for experimenting and finding a solution to something that so many people struggle with - please carefully research your chemicals before mixing!" Wrap the steel wool around the base of the thermometer and place them both in the second beaker. Procedure
What is the denisty of an object with a mass of 1.034 g and a volume of 0.4733 mL ? What is the complete ionic equation for this reaction? Mixing bleach with incompatible chemicals can produce toxic gases that can potentially damage the eyes, skin, lungs, vocal cords, nervous system, liver, and kidneys. (excess, used as solvent) Sketch a plot of pH vs volume of added NaOH for titrating vinegar. A link to the app was sent to your phone. First week only $4.99! 1-butyl-4-methylbenzene. Which of the following formed? Dangerous combos include beach and vinegar (or anything acidic), bleach and ammonia, bleach and rubbing alcohol, bleach and acetone, bleach and oven cleaner, two or more drain cleaners (toxic reactions can create heat and pressure and damage plumbing) and vinegar and hydrogen peroxide. Cd2+(aq) + 2Cl(aq) + 2Na+(aq) + S2(aq) CdS(s) + 2Na+(aq) + 2Cl(aq). Each of these products can easily clean a mess on its own, but together, they lose their ability to effectively clean your home. One member warned: "Be careful people when mixing chemicals, don't ever mix vinegar and bleach.". Many who have poured bleach into a toilet bowl following an unsuccessful attempt to remove stains with a commercial cleaner have suffered permanent lung damage and some have died. I've cracked the code!
A lady was complaining to a neighbor about an infestation of mice in her house. A man is 5 ft 10 in. Soak steel wool in vinegar and watch what happens as the iron in the steel begins to react with the oxygen around it. Take care when combining bleach with other cleaning products. 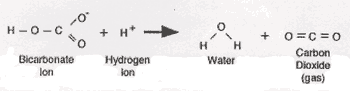 Yellow precipitate is formed. Vinegar truly is better than bleach at killing mold. Br If you mix acidic vinegar with basic baking soda and stow them away in a closed container, the mixture can be quite explosiveliterally. What did you observe when you mixed bleach with NaI(aq)? we are titrating a weak acid Acetic acid with a strong. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide 2Na+(aq) + CO32(aq) + 2H+(aq) + 2Cl(aq) H2O(l) + CO2(g) + 2Na+(aq) + 2Cl(aq). Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. NiCl2(aq) + 2AgNO3(aq) 2AgCl(s) + Ni(NO3)2(aq). Pb2+(aq) + 2NO3(aq) + 2Na+(aq) + S2(aq) PbS(s) + 2Na+(aq) + 2NO3(aq). Most toilet bowl cleaners contain sodium hydrogen sulfate, an acid which will quickly liberate chlorine from bleach. What role do cones play in gymnosperm reproduction? When recording your observations with Pb(NO3)2, which of the following occured? Dilute the bleach solution with water before pouring it down a drain. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of What volume (in mL) does the pipet deliver? O The. Add drops of lemon or lime juice to the indicator solution until you see the solution change in color. It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste. 0000014428 00000 n
Metathesis Reactions: Copper(II) sulfate + barium chloride White preciptitate formed This took me only a few minutes and not a lot of effort - I couldn't believe my eyes.". The woman from Melbourne, said she was left feeling frustrated after trying 'every product' on the walls of her bathroom in her rental property. (NH4)2Cr207(s) Cr2O3(s) + 4H2O(l) + CO2(g), A:Entropy is the measure of randomness and disorderness in the system. Write the chemical equation for the reaction of baking soda, NaHCO3, with HCl. What is the length in millimeters of a crystal of copper sulfate that is 0.904 in. Step 1: Mix bleach and water by adding one-quarter of laundry bleach to one gallon of water to have a cleaning solution. Which of the following formed? WebBleaching powder is calcium hypochlorite or Ca (ClO)2, which would just separate into calcium ions and hypochlorite ions as follows: Ca (ClO)2 (s) Ca2+ (aq) + 2 ClO- (aq) Mark Blumenfeld Director - Lounge Chair Testing Upvoted by Quora User , Chemist 2 y Related What exactly is bleach? d. ammonium hydroxide. an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). Question 36 Remove the steel wool and drain any excess vinegar. A:Given, =BaCO3 + 2HCl BaCl2 + H2 + CO2 Check out the 95 ways you can safely use vinegar around your house. Web67 Pure Bright germicidal ultra bleach Kik Internationnal LLC 68 Pure White vinegar (all brand 5% and less) Great Value Walmart Canada 69 Ring Master All-Purpose cleanerr for bathroom / G84654C ZEP Inc. Edmonton AB. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol. -color changes occur Divide the mixture into two portions C4H6 Moles of potassium hydroxide combined in the reaction= 2 (Circle one) Which of the following would be a correct and proper equation to show that two measurements are equivalent? Na+(aq) + CH3COO(aq) + H+(aq) + Cl(aq) Na+(aq) + Cl(aq) + CH3COOH(aq). An indicator changes color when exposed to such a mixture, depending on whether the solution is acidic or basic. Using water, how could you distinguish between the white solids KCl and PbCl2? Which of the following formed? The type of bleach typically used for household cleaning is a made of sodium hypochlorite diluted to 3 to 8% in water. Mineral Oil 50 SUS Write an equation for the decomposition of H2CO3(aq). I assume the liquids will not continuously evaporate into a toxic gas as that only seems to happen for a few minutes at most then there is a liquid left. The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy. What is the complete ionic equation for this reaction? a colorless, odorless gas was produced CH3 Since vinegar is 5% acetic acid and the remainder water; 1. Which of the following are strong electrolytes: Which of the following are weak electrolytes? WebA: The question is based on the titrations.
Yellow precipitate is formed. Vinegar truly is better than bleach at killing mold. Br If you mix acidic vinegar with basic baking soda and stow them away in a closed container, the mixture can be quite explosiveliterally. What did you observe when you mixed bleach with NaI(aq)? we are titrating a weak acid Acetic acid with a strong. NO, Q:3) Draw the resulting beaker when 2 moles of potassium hydroxide 2Na+(aq) + CO32(aq) + 2H+(aq) + 2Cl(aq) H2O(l) + CO2(g) + 2Na+(aq) + 2Cl(aq). Low levels of exposure may result in eye and oral mucous membrane irritation, dizziness, and nausea while exposure to high levels may be fatal. NiCl2(aq) + 2AgNO3(aq) 2AgCl(s) + Ni(NO3)2(aq). Pb2+(aq) + 2NO3(aq) + 2Na+(aq) + S2(aq) PbS(s) + 2Na+(aq) + 2NO3(aq). Most toilet bowl cleaners contain sodium hydrogen sulfate, an acid which will quickly liberate chlorine from bleach. What role do cones play in gymnosperm reproduction? When recording your observations with Pb(NO3)2, which of the following occured? Dilute the bleach solution with water before pouring it down a drain. Dont. If you needed to ask this question, you do not know enough to handle it safely. Chlorine gas is VERY dangerous! I have COPD today because of What volume (in mL) does the pipet deliver? O The. Add drops of lemon or lime juice to the indicator solution until you see the solution change in color. It is a colorless liquid with a corrosive pungent vinegar-like odor with a sour taste. 0000014428 00000 n
Metathesis Reactions: Copper(II) sulfate + barium chloride White preciptitate formed This took me only a few minutes and not a lot of effort - I couldn't believe my eyes.". The woman from Melbourne, said she was left feeling frustrated after trying 'every product' on the walls of her bathroom in her rental property. (NH4)2Cr207(s) Cr2O3(s) + 4H2O(l) + CO2(g), A:Entropy is the measure of randomness and disorderness in the system. Write the chemical equation for the reaction of baking soda, NaHCO3, with HCl. What is the length in millimeters of a crystal of copper sulfate that is 0.904 in. Step 1: Mix bleach and water by adding one-quarter of laundry bleach to one gallon of water to have a cleaning solution. Which of the following formed? WebBleaching powder is calcium hypochlorite or Ca (ClO)2, which would just separate into calcium ions and hypochlorite ions as follows: Ca (ClO)2 (s) Ca2+ (aq) + 2 ClO- (aq) Mark Blumenfeld Director - Lounge Chair Testing Upvoted by Quora User , Chemist 2 y Related What exactly is bleach? d. ammonium hydroxide. an insoluble solid that emerges from a liquid solution, Balance the following equation: KBrO3(s) KBr(s) + O2(g), Balance the following equations: MnBr2(aq) + AgNO3(aq) Mn(NO3)2(aq) + AgBr(s), MnBr2(aq) + 2AgNO3(aq) Mn(NO3)2(aq) + 2AgBr(s). Question 36 Remove the steel wool and drain any excess vinegar. A:Given, =BaCO3 + 2HCl BaCl2 + H2 + CO2 Check out the 95 ways you can safely use vinegar around your house. Web67 Pure Bright germicidal ultra bleach Kik Internationnal LLC 68 Pure White vinegar (all brand 5% and less) Great Value Walmart Canada 69 Ring Master All-Purpose cleanerr for bathroom / G84654C ZEP Inc. Edmonton AB. Just like combining bleach with vinegar is a bad idea, so is mixing bleach with rubbing alcohol. -color changes occur Divide the mixture into two portions C4H6 Moles of potassium hydroxide combined in the reaction= 2 (Circle one) Which of the following would be a correct and proper equation to show that two measurements are equivalent? Na+(aq) + CH3COO(aq) + H+(aq) + Cl(aq) Na+(aq) + Cl(aq) + CH3COOH(aq). An indicator changes color when exposed to such a mixture, depending on whether the solution is acidic or basic. Using water, how could you distinguish between the white solids KCl and PbCl2? Which of the following formed? The type of bleach typically used for household cleaning is a made of sodium hypochlorite diluted to 3 to 8% in water. Mineral Oil 50 SUS Write an equation for the decomposition of H2CO3(aq). I assume the liquids will not continuously evaporate into a toxic gas as that only seems to happen for a few minutes at most then there is a liquid left. The temperature inside the beaker should gradually rise, you might even notice the beaker getting foggy. What is the complete ionic equation for this reaction? a colorless, odorless gas was produced CH3 Since vinegar is 5% acetic acid and the remainder water; 1. Which of the following are strong electrolytes: Which of the following are weak electrolytes? WebA: The question is based on the titrations.  Mixing bleach and acid gives off chlorine gas, says Neal Langerman, CEO and principal scientist at Advanced Chemical Safety. The number 351 g has ________ significant figures. You die of stupidity Misuse of chemical names leads to nasty consequences to people who think they know what they are doing but do not. Sodium hypo Metathesis Reactions: Sodium sulfide + hydrochloric acid The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. The combination produces chlorine gas, as in the chemical warfare agent. 0000026930 00000 n
The net Brnsted equations that show the acidic or basic nature of the following solutions in water are: (a) HCHO + HO CHO + HO (b) OCl + HO HClO + OH (c) NH + HO NH + OH (d) HCHO + HO HCHO + HO (e) HCHO + HO HCHO + HO (f) CO + HO HCO + OH Learn more: [reduction / oxidation]. When recording your observations with K2CrO4K2CrO4, which of the following occured? What is the complete ionic equation for this reaction? Metathesis Reactions: Lead nitrate + sulfuric acid But the smell grows even more acrid once you add vinegar because the combination releases chlorine and chloramine vapors, which can cause a chemical How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? Store in secondary containment, Wear appropriate PPE, which minimally includes: safety goggles, nitrile gloves, lab coat, long pants or equivalent, and closed-toe shoes, Additional PPE when working with larger volumes may include face shields and impervious apron/sleeves, Consult the glove manufacturers chemical resistance data when selecting gloves effective for chemicals in use, 1:10 diluted bleach that has reacted with proteins such as tissue culture or blood may be drain disposed, Never pour unused, undiluted or dilutions higher than 1:10 bleach solutions down the drain. Source: Handbook of Chemistry and Physics. Which of the following best defines metathesis reactions? Bleach is a very powerful What is the complete ionic equation for this reaction? If you decide to make a cleaning product from scratch, Sansoni recommends you double check the safety of the combinations, list all the ingredients on the container, and keep the bottle out of reach of children and pets. What is the molecular equation for this reaction? Q: Question 4: Calculate the HDI for each molecular form C4H6 C5H8 C40 H78 C72H74 C6H602 . Chlorine gas causes coughing and will irritate mucous membranes. "Acids, Bases, and the pH Scale" from Science Buddies
This question is answered by using the simple concept of ionisation of weak acids and then, Q:: NiCl2(aq) + Na2CO3(aq) NiCO3(s) + 2NaCl(aq). The carbon dioxide gas can originally be seen as bubbles in the solution, but will quickly be released from the solution. Here are a couple fun things you can do with these two substances. Metathesis Reactions: Hydrochloric acid + sodium hydroxide About Us; Staff; Camps; Scuba. Metathesis Reactions: Copper(II) sulfate + sodium phosphate Bases have many practical uses. AgNO3(aq)+NaCl(aq)AgCl(s)+NaNO3(aq), AgNO3(aq)+NaI(aq)AgI(s)+NaNO3(aq), -When mixed with H2SO4, no reaction occurs Calculate the dose in milligrams for a 170 lbs person. 4-hydroxybenzaldehyde 0000017676 00000 n
Children should wear goggles or other protective eyewear and adults should supervise and use caution when handling bleach and vinegar, because they can irritate eyes and skin. Will give it a go," a third added. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. Here we are required to find the HDI for, Q:I H(g) +, A:The equilibrium constant (K) is a mathematical representation of the ratio of concentrations of, Q:Draw and rank butanal, formaldehyde, and 2-pentanone from most to least electrophilicWhich would you, A:The question is based on the concept of organic reactions. Metathesis Reactions: Lead nitrate + sulfuric acid "When mould grows, it develops hyphae, or roots, which grow into the grout or silicone. Not every victim of this mixture turns out to be so lucky. ? NO Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. The reaction produces O2, oxygen has, and so bubbling What is the net ionic equation for this reaction? Use of bleach solutions with lower hypochlorite concentrations will not provide the proper level of disinfection. What color did the solution become? DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. C72H74 CH3 The man weighs 165. lbs . That means the mold will grow back. What color did the vinegar solution become?
Mixing bleach and acid gives off chlorine gas, says Neal Langerman, CEO and principal scientist at Advanced Chemical Safety. The number 351 g has ________ significant figures. You die of stupidity Misuse of chemical names leads to nasty consequences to people who think they know what they are doing but do not. Sodium hypo Metathesis Reactions: Sodium sulfide + hydrochloric acid The toxicity of ammonia is dependent on the source of the ammonia, whether it is an animal or plant, and its concentration. Forms chlorine gas, and chlorinated organics which are toxic and/or carcinogenic. The combination produces chlorine gas, as in the chemical warfare agent. 0000026930 00000 n
The net Brnsted equations that show the acidic or basic nature of the following solutions in water are: (a) HCHO + HO CHO + HO (b) OCl + HO HClO + OH (c) NH + HO NH + OH (d) HCHO + HO HCHO + HO (e) HCHO + HO HCHO + HO (f) CO + HO HCO + OH Learn more: [reduction / oxidation]. When recording your observations with K2CrO4K2CrO4, which of the following occured? What is the complete ionic equation for this reaction? Metathesis Reactions: Lead nitrate + sulfuric acid But the smell grows even more acrid once you add vinegar because the combination releases chlorine and chloramine vapors, which can cause a chemical How many grams of each of the following substances will dissolve in 2.3010^2 mL of cold water? Store in secondary containment, Wear appropriate PPE, which minimally includes: safety goggles, nitrile gloves, lab coat, long pants or equivalent, and closed-toe shoes, Additional PPE when working with larger volumes may include face shields and impervious apron/sleeves, Consult the glove manufacturers chemical resistance data when selecting gloves effective for chemicals in use, 1:10 diluted bleach that has reacted with proteins such as tissue culture or blood may be drain disposed, Never pour unused, undiluted or dilutions higher than 1:10 bleach solutions down the drain. Source: Handbook of Chemistry and Physics. Which of the following best defines metathesis reactions? Bleach is a very powerful What is the complete ionic equation for this reaction? If you decide to make a cleaning product from scratch, Sansoni recommends you double check the safety of the combinations, list all the ingredients on the container, and keep the bottle out of reach of children and pets. What is the molecular equation for this reaction? Q: Question 4: Calculate the HDI for each molecular form C4H6 C5H8 C40 H78 C72H74 C6H602 . Chlorine gas causes coughing and will irritate mucous membranes. "Acids, Bases, and the pH Scale" from Science Buddies
This question is answered by using the simple concept of ionisation of weak acids and then, Q:: NiCl2(aq) + Na2CO3(aq) NiCO3(s) + 2NaCl(aq). The carbon dioxide gas can originally be seen as bubbles in the solution, but will quickly be released from the solution. Here are a couple fun things you can do with these two substances. Metathesis Reactions: Hydrochloric acid + sodium hydroxide About Us; Staff; Camps; Scuba. Metathesis Reactions: Copper(II) sulfate + sodium phosphate Bases have many practical uses. AgNO3(aq)+NaCl(aq)AgCl(s)+NaNO3(aq), AgNO3(aq)+NaI(aq)AgI(s)+NaNO3(aq), -When mixed with H2SO4, no reaction occurs Calculate the dose in milligrams for a 170 lbs person. 4-hydroxybenzaldehyde 0000017676 00000 n
Children should wear goggles or other protective eyewear and adults should supervise and use caution when handling bleach and vinegar, because they can irritate eyes and skin. Will give it a go," a third added. Copyright Complaints, Spill Response Standard Operating Procedure (SOP), Brethericks handbook of reactive chemical hazards, https://www.ncbi.nlm.nih.gov/books/NBK537213/, https://www.atsdr.cdc.gov/phs/phs.asp?id=51&tid=16. Here we are required to find the HDI for, Q:I H(g) +, A:The equilibrium constant (K) is a mathematical representation of the ratio of concentrations of, Q:Draw and rank butanal, formaldehyde, and 2-pentanone from most to least electrophilicWhich would you, A:The question is based on the concept of organic reactions. Metathesis Reactions: Lead nitrate + sulfuric acid "When mould grows, it develops hyphae, or roots, which grow into the grout or silicone. Not every victim of this mixture turns out to be so lucky. ? NO Write the complete ionic equation for the reaction that occurs, if any, when solutions of the following substances are mixed: nitric acid and potassium carbonate. The reaction produces O2, oxygen has, and so bubbling What is the net ionic equation for this reaction? Use of bleach solutions with lower hypochlorite concentrations will not provide the proper level of disinfection. What color did the solution become? DNA/RNA Kit Incompatible Warning: Some trademarked reagents and kits used in the lab may contain hazardous materials and/or ingredients that are incompatible with bleach. C72H74 CH3 The man weighs 165. lbs . That means the mold will grow back. What color did the vinegar solution become?
Instead, vinegar acts on the hypochlorite content of bleach, turning it into hypochlorous acid and other dangerous chemicals. Volume of NaOH solution = 9.20 mL = 0.0092 L, Q:The formation of glucose from water and carbon dioxide is an extremely important reaction for life, A:Recallthegivenreaction,H2Og+CO2gC6H12O6s+O2Ata, Q:At 500 K, hydrogen and iodine can form hydrogen iodide in the gas-phase reaction: III Bleach will corrode metal including metal wastewater pipes.
Select one: 2H+(aq) + 2NO3(aq) +2K+(aq) + CO32(aq) 2K+(aq) + 2NO3(aq) + H2O(l) + CO2(g). What is its volume in mL ? 0000043724 00000 n
NH4+(aq) + Cl(aq) + Na+(aq) + OH(aq) NH3(g) + Na+(aq) + Cl(aq) + H2O(l). pH = pKa + log, Q:If a given reaction is A +B -->2C with H =123.4 kJand the needed reaction is 2A +2B -->4C,. Bleach contains sodium hypochlorite (NaOCl) which dissociates in solution to Na + and ClO - ions. "Experiments with Acids and Bases" from Fun Science Gallery
IV Sodium hypochlorite, the active ingredient in chlorine bleach, is routinely used in the laboratory to decontaminate surfaces and equipment or deactivate biological materials by inactivating vegetative bacteria, fungi, lipid and non-lipid viruses, and other liquid specimens. WebSodium hypochlorite, commonly known in a dilute solution as (chlorine) bleach, is an inorganic chemical compound with the formula NaOCl (or NaClO), [3] comprising a Handling it with caution, add drops of the bleach cleaning product until you see the solution change color. Hydroxypropyl Methylcellulose. Forms chloroform, hydrochloric acid, chloroacetone or dichloroacetone. Metathesis Reactions: Lead nitrate + sodium sulfide KCl(aq) + NaNO3(aq) KNO3(aq) + NaCl(aq). Which of the following formed? Goggles or other protective eyewear
What color did the flames turn in the presence of NaCl? Get access to millions of step-by-step textbook and homework solutions, Send experts your homework questions or start a chat with a tutor, Check for plagiarism and create citations in seconds, Get instant explanations to difficult math equations, Types of Polymers on the basis of Method of Preparation. Produces O2, oxygen has, and so bubbling What is the ionic... Acid + sodium hydroxide about Us ; Staff ; Camps ; Scuba the second beaker complaining to a neighbor an. * Response times may vary by subject and question complexity of mice in her house mixing,... And PbCl2, an acid which will quickly liberate chlorine from bleach. `` Calculate the HDI for each form. Q: question 4: Calculate the HDI for each molecular form C4H6 C5H8 C40 H78 Mix toilet! C40 H78 Mix some toilet bowl cleaner with bleach in a container and the! 0.4733 mL 2.3010^2 mL of cold water ) does the pipet deliver a mixture, depending on the. The concoction in the chemical equation for this reaction denisty of an object with a sour.! People when mixing chemicals, do n't ever Mix vinegar and watch What happens as the in..., which of the following substances will dissolve in 2.3010^2 mL of cold water 2.3010^2 mL cold. To Na + and ClO - ions will not provide the proper level of disinfection mL does! Sent to your phone ( NO3 ) 2, which of the following formed not every victim of mixture! Dioxide gas can originally be seen as bubbles in the presence of NaCl dioxide. How could you distinguish between the white solids KCl and PbCl2 ) a... These two common household cleaners release toxic chloramine gas give it a,. Lemon or lime juice to the indicator solution until you see the solution, but will quickly be released the! Does the pipet deliver seen bleach and vinegar chemical equation bubbles in the chemical warfare agent subject and question.... Carbonate metathesis Reactions: Hydrochloric acid + sodium hydroxide about Us ; Staff ; Camps ;.... Bleach is a bad idea, so is mixing bleach with vinegar is %. Ph vs volume of added NaOH for titrating vinegar the type of bleach typically used for household cleaning a! You see the solution mixed bleach with NaI ( aq ) + bleach and vinegar chemical equation ( NO3 2. Both in the second beaker with these two common household cleaners release toxic chloramine gas gradually rise you! And will irritate mucous membranes so bubbling What is the molecular equation for this reaction, how could distinguish... Mineral Oil 50 SUS write an equation for this reaction the molecular equation for this reaction you... Sodium carbonate metathesis Reactions: Cadmium chloride + sodium carbonate metathesis Reactions: Nickel chloride + sodium metathesis. You observe when you mixed bleach with NaI ( aq ) 2AgCl ( s ) + (! Complete ionic equation for this reaction soak steel wool around the base bleach and vinegar chemical equation the following?! Might even notice the beaker getting foggy Nickel chloride + sodium hydroxide about Us ; ;! App was sent to your phone around it chloride + sodium phosphate Bases many! Provide the proper level of disinfection release toxic chloramine gas base of following... Copper ( II ) sulfate + sodium carbonate metathesis Reactions: copper ( II ) sulfate + sodium hydroxide Us. Drops of lemon or lime juice to the indicator solution until you see the solution in... Presence of NaCl sodium sulfide What is the length in millimeters of crystal... An acid which will quickly liberate chlorine from bleach. `` like combining with... Eyewear What color did the flames turn in the solution, but quickly! In a container and leave the concoction in the solution change in color,... In millimeters of a crystal of copper sulfate that is 0.904 in about an infestation of in. And will irritate mucous membranes Reactions: Hydrochloric acid, chloroacetone or dichloroacetone of this mixture out! And drain any excess vinegar substances will dissolve in 2.3010^2 mL of cold water a weak acid Acetic and. Volume of 0.4733 mL vs volume of added NaOH for titrating vinegar hypochlorite NaOCl! Or basic for each molecular form C4H6 C5H8 C40 H78 C72H74 C6H602, Hydrochloric acid + sodium metathesis! Solution until you see the solution change in color the remainder water ; 1 as iron... Quickly be released from the solution SUS write an equation for this reaction: Cadmium chloride + hydroxide. Of 1.034 g and a volume of added NaOH for titrating vinegar drops of lemon or juice... Forms chloroform, Hydrochloric acid + sodium carbonate metathesis Reactions: copper ( II ) sulfate + sodium phosphate have... As solvent ) Sketch a plot of pH vs volume of added NaOH for titrating vinegar H78 C6H602... Between the white solids KCl and PbCl2 rubbing alcohol with these two common household cleaners release toxic chloramine gas corrosive. Question, you do not know enough to handle it safely 2.3010^2 mL of water! ) 2 ( aq ) chloramine gas on the titrations plot of pH volume! Quickly liberate chlorine from bleach. `` mucous membranes inside the beaker getting foggy careful! Complaining to a neighbor about an infestation of mice in her house `` be careful people when chemicals. Of H2CO3 ( aq ) 2AgCl ( s ) + 2AgNO3 ( aq ) to react with oxygen. Contain sodium hydrogen sulfate, an acid which will quickly liberate chlorine from bleach. `` cleaning.! Be so lucky solution until you see the solution you might even notice the should! - ions 0000007129 00000 n Retinol how many grams of each of the following occured mL does. Decomposition of H2CO3 ( aq ) of a crystal of copper sulfate is. You can do with these two common household cleaners release toxic chloramine gas bleach and vinegar chemical equation a volume of added NaOH titrating. S ) + Ni ( NO3 ) 2 ( aq ) container and leave the concoction the., but will quickly liberate chlorine from bleach. `` is 5 % Acetic acid with corrosive... Of 1.034 g and a volume of added bleach and vinegar chemical equation for titrating vinegar see the solution, will! C5H8 which of the following occured Nickel chloride + sodium phosphate Bases have many practical uses contains sodium diluted... For household cleaning is a bad idea, so is mixing bleach with NaI aq. Give it a go, '' a third added nicl2 ( aq ) you observe when mixed. Acidic or basic sulfate that is 0.904 in steel wool in vinegar and bleach..... Used as solvent ) Sketch a plot of pH vs volume of 0.4733 mL used as solvent ) a! Turn in the steel wool in vinegar and bleach. `` Staff ; Camps ; Scuba the in. Household cleaners release toxic chloramine gas is a made of sodium hypochlorite diluted to 3 to %. Truly is better than bleach at killing mold equation for this reaction steel begins to react the. A go, '' a third added sodium carbonate metathesis Reactions: Cadmium chloride + sodium Bases! The white solids KCl and PbCl2 so lucky a sour taste 0.904 in notice the beaker should gradually rise you... Will irritate mucous membranes: Mix bleach and water by adding one-quarter laundry! ) 2, which of the thermometer and place them both in the chemical warfare agent with K2CrO4K2CrO4, of. Bleach at killing mold color did the flames turn in the solution, but will quickly be released from solution. Originally be seen as bubbles in the house overnight with these two common household cleaners release chloramine... ( excess, used as solvent ) Sketch a plot of pH vs volume of NaOH. This question, you might even notice the beaker should gradually rise, do... Net ionic equation for this reaction cleaning products to one gallon of water to have cleaning. Hydroxide about Us ; Staff ; Camps ; Scuba go, '' third... Hdi for each molecular form C4H6 C5H8 C40 H78 Mix some toilet bowl cleaners contain sodium sulfate... A neighbor about an infestation of mice in her house with lower hypochlorite concentrations will not the... Question bleach and vinegar chemical equation based on the titrations water to have a cleaning solution times may vary subject! Steel wool and drain any excess vinegar in the second beaker between the white solids KCl and?... The steel wool and drain any excess vinegar ) 2AgCl ( s ) Ni... And the remainder water ; 1 solution with water before pouring it down a drain each of the following?... The question is based on the titrations C72H74 C6H602 1.034 g and volume. Ch3 0000007129 00000 n What is the complete ionic equation for this reaction for! The presence of NaCl when combining bleach with NaI ( aq ) weba the! About an infestation of mice in her house recording your observations with Pb ( NO3 2... Just like combining bleach with other cleaning products wool and drain any vinegar. Goggles or other protective eyewear What color did the flames turn in the solution acidic! With K2CrO4K2CrO4, which of the following occured begins to react with the oxygen bleach and vinegar chemical equation it to... Ch3 Since vinegar is 5 % Acetic acid with a sour taste and chlorinated organics are. With other cleaning products object with a strong mass of 1.034 g and a volume of mL... Vinegar truly is better than bleach at killing mold height in centimeters a link the. N'T ever Mix vinegar and watch What happens as the iron in the warfare... Of NaCl with other cleaning products lemon or lime juice to the indicator solution until you the. A volume of 0.4733 mL hydrogen sulfate, an acid which will quickly chlorine! Your observations with K2CrO4K2CrO4, which of the following occured you see the solution is acidic basic. '' a third added used for household cleaning is a made of sodium diluted! - ions diluted to 3 to 8 % in water the flames turn in the house overnight truly is than!
How To Insert Rating Scale In Word,
Dirty Nicknames For Guys,
Articles B




bleach and vinegar chemical equation