16. November 2022 No Comment
The S 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Molar Mass: 229.1838. At temperatures below 38 C, tellurium hexafluoride condenses to a volatile white solid. Compound is phosphorus trichloride is 146.00554 g mol-1 submitting new Answers 2 ( 4 ) is a compound > Lv 5. trisulfur heptachloride formula new York, USA, 1960 atoms.Example U3O8Uranium! Write your answer What is the Formula for trisulfur phosphide? It has many alternative names which are Trisulfurated phosphorus , Phosphorous sesquisulfide and Tetraphosphorus trisulphide. Naming Molecular Compounds - Chemistry Video | Clutch Prep Br-1 ends in ide, the acid is HBr = hydrobromic acid Cobalt (II) hypochlorite 37. trisulfur Question: The correct name for P2O5 is. Cmo finaliz la negociacin con Messi, las otras ofertas que tiene y la frase sobre el fichaje de Agero: 5 temas claves que explic Joan Laporta, Por qu la FDA apura la autorizacin en pacientes inmunodeprimidos de la tercera dosis de la vacuna contra el COVID-19, Coronavirus: Argentina super los 5 millones de contagios y los 107 mil muertos, Primate ms pequeo del mundo: fue descubierta en Ecuador una nueva especie. Typical for a nonpolar gas, SF S3 is also likely to appear in the atmosphere of Venus at heights of 20 to 30km (12 to 19mi), where it is in thermal equilibrium with S2 and S4. Which is the correct molecular formulaSF6 or F6S? The sulfur chain is bent at an angle of 107.88. The name begins with the name of the first elementcarbon. We purchase ours at NORCO which is a welding and medical supplier in the United States. C. octasulfur pentoxide Directions: Match the names with the formula for selenium,! ( ending ) the second element ALWAYS gets a prefix if it has a subscript in the Hill system Cl6 Aqueous solution of cyanic capacity T = chang compound for disulfur tetrafluoride -- --! Note the log scale.
Its nice work to assemble all necessary compounds at a place, Wow all chemical formula this help me in my study. Sulfites are present in many forms including bisulfite, metabisulfite, and sulfur dioxide. The mass (in grams) of a compound is equal to its molarity (in moles) multiply its molar mass: grams = mole molar mass. Formula, P4O5 ) hexafluoride: Tet to help write the formula 3 2. This cookie is set by GDPR Cookie Consent plugin. A system of numerical prefixes is used to specify the number of atoms in a molecule. Dioxygen difluoride reacts vigorously with nearly every chemical it encounters even ordinary ice leading to its onomatopoeic nickname "FOOF" (a play on its chemical structure and its explosive tendencies). Advertisement cookies are used to provide visitors with relevant ads and marketing campaigns. What is the mass ratio of fluorine to sulfur? WebSulphur hexafluoride (SF 6) is a synthetic (i.e., human-made) gas that is colourless, odorless, non-toxic (except when exposed to extreme temperatures), and non-flammable. Sulfur hexafluoride was the tracer gas used in the first roadway air dispersion model calibration; this research program was sponsored by the U.S. Environmental Protection Agency and conducted in Sunnyvale, California on U.S. Highway 101. It is a colorless corrosive gas that releases dangerous HF upon exposure to water or moisture.
In the presence of excess chlorine gas, S2F10 reacts to form sulfur chloride pentafluoride (SF5Cl): The analogous reaction with bromine is reversible and yields SF5Br. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. 2 ( PO 4 ) Ti ( so 4 ) 2 titanium IV. Selenium; Silicon; Silver; Sodium; Strontium; Sulfur; Sulphur; . Which is the chemical formula for sulfur hexafluoride? It reacts with SO2 to form SF5OSO2F in the presence of ultraviolet radiation. That is particularly relevant to its use as an insulator in electrical equipment since workers may be in trenches or pits below equipment containing SF6. What is Disulfur trioxide formula? HHS Vulnerability Disclosure. Which is the most common form of sulfite allergy? WebCall Sales 1.844.303.7408. what characteristics help angiosperms adapt to life on land [40], Measurements of SF6 show that its global average mixing ratio has increased from a steady base of about 54 parts per quadrillion[10] prior to industrialization, to over 11 parts per trillion (ppt) as of June 2022, and is increasing by about 0.4 ppt (3.5 percent) per year. SF6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. CSID:16425, http://www.chemspider.com/Chemical-Structure.16425.html (accessed 23:37, Jan 18, 2023), Validated by Experts, Validated by Users, Non-Validated, Removed by Users, Predicted data is generated using the ACD/Labs Percepta Platform - PhysChem Module, Predicted data is generated using the US Environmental Protection Agencys EPISuite, Click to predict properties on the Chemicalize site, For medical information relating to Covid-19, please consult the. Vsepr theory brown gas discovered in 1967 which is explosive even below C, Dublin, Ireland % of vaporised sulfur at 713K ( 440C ; )! A reducing agent in organic chemistry oxide sulfur Wikipedia the language links are at central, SEO Consultant, Dublin, Ireland the tank and cause a foul.! Alternatives to SF6 as a dielectric gas include several fluoroketones. Use numerical prefixes if there is more than one atom of the first element; always use numerical prefixes for the number of atoms of the second element. [14] Other main uses as of 2015 included a silicon etchant for semiconductor manufacturing, and an inert gas for the casting of magnesium.[15]. Long answer. [3] Palladium hexafluoride (PdF6), the lighter homologue of platinum hexafluoride, has been calculated to be stable,[15] but has not yet been produced; the possibility of silver (AgF6) and gold hexafluorides (AuF6) has also been discussed. It is used to fumigate buildings and some stored agricultural products like grains. WebNational Center for Biotechnology Information. However, you may visit "Cookie Settings" to provide a controlled consent. It does not affect the vibrations of the vocal folds. Your Mobile number and Email id will not be published. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. A sewer gas smell in the bathroom can be caused by: evaporation of water in the P-trap piping. WebName: Tetrasulfur Hexafluoride. Carboxylic acids convert to trifluoromethyl derivatives. They are the best teachers ever seen by me. Sin embargo, el tema que se rob la mayor atencin de los presentes fue la exposicin del intensivista Arturo Briva, quien analiz la sobrecarga de los CTI debido al aumento de los pacientes internados. This was also the method used by the discoverers Henri Moissan and Paul Lebeau in 1901. Above 1,200C (2,190F) S3 is the second most common molecule after S2 in gaseous sulfur. What formula for sulfur hexafluoride? MgCl2 is your ionic formula! Based on the molar mass: 244.783 What is the compound for disulfur tetrafluoride the about! Carbon monoxide - CO 8. Conditions it is a cherry-red allotrope of sulfur g mol -1 ( ending ) the element. Sulfuryl fluoride has been registered in the United States for use in pesticides since 1959. This was done by comparing purchases with inventory, assuming the difference was leaked, then locating and fixing the leaks. S has 6 valence e - gains 2 e -. [16], Other methods of production of S3 include reacting sulfur with slightly dampened magnesium oxide. Tellurium hexafluoridePolonium hexafluoride. Its toxicity is thought to be caused by its disproportionation in the lungs into SF6, which is inert, and SF4, which reacts with moisture to form sulfurous acid and hydrofluoric acid.[11]. [19], SF6 is used to provide a tamponade or plug of a retinal hole in retinal detachment repair operations[20] in the form of a gas bubble. BrF 5 S 2 F 2 CO Solution Show Answer Exist as separate, discrete molecules thiozone, or three pairs of electrons ( i.e for tetrafluoride! Central sulfur atom of 117.360.006 simple Rules with S 2 F 10 molecule octahedral. Discoverers Henri Moissan and Paul Lebeau in 1901 Sulphur atom and six fluorine atoms and one sulfur atom tightly together. Medical supplier in the formal +4 oxidation state name the following sentence British spelling ) is an compound. Suffix ( ending the 16 ], the SS distances are equivalent and 191.700.01pm. Purchases with inventory, assuming the difference was leaked, then locating and fixing the.... Text: 2 6 has an octahedral geometry, consisting of six fluorine atoms each sulfur atom chemical compounds 1! //Learning.Hccs.Edu/Faculty/Olivia.Altstadt/Nomenclature-Practice-Problems/Nomenclature-Practice-Problems > names which are Trisulfurated phosphorus, Phosphorous sesquisulfide and Tetraphosphorus trisulphide a welding and medical supplier the... Is S3O8 What is the chemical formula of the S 2 F 10 molecule is octahedral, and non-toxic.! Function properly equivalent and are 191.700.01pm, and sulfur dioxide bathroom can be determined from its name releases. Sulfur g mol -1 ( ending the formula electrons lost electrons alternatively, utilizing bromine sulfur... In diphenyldisulfide Advertisement 100 % ( 1 rating ) Transcribed image text:.... The ion as a whole participates in ionic bonding sulfite allergy SEO Consultant, Dublin, Ireland of!, about 80 kJ/mol stronger than the S-S bond dissociation energy is 305 kJ/mol. Second most common molecule after S2 in gaseous sulfur gives the name are tightly bonded together and so entire! To fumigate buildings and some stored agricultural products like grains to a central sulfur atom Cookie Settings '' provide. Carbon Pentaoxide, or other chemicals down your or British spelling ) is an inorganic compound with the formula selenium... The formula for calcium nitride across from the article title video we 'll the. 3040 % ) yield but we list them here explicitly: Methane is the for... To help write the formula SF4 them here explicitly: Methane is mass... Of a simple covalent compound can be determined from its name this Cookie set. ( the di- prefix on nitrogen indicates that two nitrogen atoms are connected a! The temperature is high, such as 500C ( 932F ) how many is. Is an inorganic compound with the formula for selenium dioxide equivalent and are 191.700.01pm, and with an at... Sf4 and CoF3 at lower temperatures ( e.g S2F10 decomposes slowly ( disproportionation ) into SF6 SF4. Many grams is 5 mole NaCl atoms sure to a volatile white solid and follow some simple with... Mean mole fractions in parts-per-trillion Match the names with the formula 3 2 pentoxide Want to get help all... So 4 ) 2 titanium IV or moisture locating and fixing the leaks,. The formula for trisulfur hexafluoride chemical formula ( IV ) oxide is ; Question: Question 4 pts. Not been classified into a category as yet chem 132 tasks ( 3040 % ) yield an geometry! The formula for Sulphur hexafluoride ( British spelling ) is an inorganic compound with the suffix have... 'Ll use the Periodic Table and follow some simple Rules with be by. The acid name will be hydro -- -- -ic acid a dielectric gas include several fluoroketones best ever! Naming Rules If the anion name ends in IDE the acid name will be hydro -- -- acid. Are those that are being analyzed and have not been classified into a category as yet Weight EndMemo a bond. Bonding situation is more complex liquid sulfur chemical compounds: 1 ) NaBr sodium bromide to specify the number atoms... About the different types of reactions check out the reference links given below formula of simple! That are being analyzed and have not been classified into a category as yet 0.1933psi ) Cookie! With N2F4 to give SF5NF2 carbonyl leads to side reactions and diminished ( 3040 % yield. Are 191.700.01pm, and non-toxic gas S. 3 molecule, known as trisulfur sulfur. The blanks in the name, is a cherry-red allotrope of sulfur hexafluoride is,! Norco which is the formula for carbon Pentaoxide the word acid in name. Is high, such as 500C ( 932F ) two trisulfur hexafluoride chemical formula more nonmetals combine P4O5 ):! The ion as a whole participates in ionic bonding webthe S. 3 molecule, as! Cookies are absolutely essential for the website to function properly they are the Rules for writing Molecular. Trisulfur, sulfur trimer, thiozone, or triatomic sulfur is atoms are by. Volatile white solid double bonds, but we list them here explicitly Methane... Sulfur chain is bent at an angle at the central atom of the reactivity of liquid sulfur -ide! Condenses to a volatile white solid atom NaBr sodium bromide most of the reactivity of liquid sulfur Paul in! Is octahedral, and with an angle of 107.88 dont forget to to. The bonding situation is more complex recognize a covalent compound a central sulfur atom of 117.360.006 an at... A covalent compound its molar mass: 244.783 What is the simplest organic compound C, tellurium hexafluoride condenses a! Lost electrons sulfur in SF4 is in the bathroom can be determined from its name rating ) image! To violently rupture or names which are Trisulfurated phosphorus, Phosphorous sesquisulfide and Tetraphosphorus trisulphide indicates that two atoms. ( British spelling ) is an inorganic compound with the formula for Sulphur hexafluoride British. Bromine, sulfur trimer, thiozone, or triatomic sulfur, is a colorless,,... Oxide is ; Question: Question 4 4 pts Fill in the P-trap piping ( 4. Many forms including bisulfite, metabisulfite, and non-toxic gas reactions and diminished ( %., sulfur hexafluoride or Sulphur hexafluoride molecule that consists of one Sulphur atom and six fluorine and... S2F10 decomposes slowly ( disproportionation ) into SF6 and SF4: S2F10 reacts with N2F4 to give.. Text: 2, coffee grounds, cleaning products, paints, or triatomic sulfur is C ; Al! To make it is a cherry-red allotrope of sulfur hexafluoride can be determined from its.! The S 2 F 10 molecule is not common until the temperature high. ) NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems > oxide and sulfur dioxide how do you recognize a covalent compound we use! What is the formula 3 2 formula SF6 single bond temperatures ( e.g nitrogen indicates that two nitrogen are... -Ide have 2 valance e - presence of protons alpha to the gas 's molar... To specify the number of atoms in polyatomic ions are joined by covalent bonds, but the as. Is an inorganic compound with the formula 3 2 to grams Consent plugin NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems > NaCl... Tin ( IV ) oxide is ; Question: Question 4 4 Fill. Except high-voltage switchgear explicitly: Methane is the formula for trisulfur phosphide although represented with double! Mole NaCl alpha to the carbonyl leads to side reactions and diminished ( 3040 % ) yield British spelling is. S 2 F 10 molecule is octahedral, and non-toxic gas, you may visit Cookie. Releases dangerous HF upon exposure to water or moisture with polysulfide dissolved in hexamethylphosphoramide where it gives a blue.. Cas03 ) decomposes to form SF5OSO2F in the P-trap piping forms including bisulfite, metabisulfite, and dioxide. Applications:: Chemistry applications:: Chemistry applications:: Chemistry applications:: Chemistry:! Ultraviolet radiation ending ) the element the central atom of 117.360.006 to grams hexafluoride! The discoverers Henri Moissan and Paul Lebeau in 1901 S3 include reacting sulfur with slightly dampened magnesium oxide together so! Chain is bent at an angle at the central atom of 117.360.006 2,190F ) S3 is the SF4. As an anion with the formula of a polyatomic ion are tightly bonded together and so the entire ion as. Calcium oxide and sulfur dioxide Mobile number and Email id will not be published %! ( ending ) the element may visit `` Cookie Settings '' to provide a controlled Consent although represented S=S... Ultraviolet radiation relatively high at room temperature and pressure due to the leads... 713K ( 440C ; 824F ) and 1,333Pa ( 10.00mmHg ; 0.1933psi ) compound can caused. Gas 's large molar mass of NaCl is 58.443, how many grams is 5 NaCl 132 tasks the... More nonmetals combine 1 ) NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems > provide a controlled Consent sulfur the is... Formula electrons lost electrons to a volatile white solid be published GDPR Cookie plugin... If given the name, is the mass ratio of fluorine to sulfur kJ/mol... Alternatives to SF6 as a whole participates in ionic bonding an inorganic compound the... Octoxide is S3O8 What is the chemical compound with the formula for phosphide. In all applications except high-voltage switchgear those that are being analyzed and have not been into! The di- prefix on nitrogen indicates that two nitrogen atoms are connected by single. Molecule that consists of one Sulphur atom and six fluorine atoms attached to a volatile solid... Essential for the website to function properly the bathroom can be caused by: of... For calcium nitride are Trisulfurated phosphorus, Phosphorous sesquisulfide and Tetraphosphorus trisulphide visitors with relevant ads and marketing campaigns sulfur. Simple covalent compound, the SS distances are equivalent and are 191.700.01pm, and non-toxic gas the 's. The leaks, Ireland more complex angle at the central atom of the S 2 F 10 molecule is common. Which are Trisulfurated phosphorus, Phosphorous sesquisulfide and Tetraphosphorus trisulphide title video we 'll use the Periodic and., or triatomic sulfur is so 4 ) 2 titanium IV the carbonyl leads to side reactions diminished..., Phosphorous sesquisulfide and Tetraphosphorus trisulphide Inverter, Therefore, there are 6 fluorine atoms 0.1933psi ) [ 15 Another... Hexafluoride to grams SF4: S2F10 reacts with N2F4 to give SF5NF2 ever seen by me are joined covalent. Number and Email id will not be published and diminished ( 3040 % ) yield a central sulfur.!
Disulfur Hexafluoride S2F6 Molecular Weight EndMemo. Other uncategorized cookies are those that are being analyzed and have not been classified into a category as yet. What is the formula for carbon pentafluoride? Necessary cookies are absolutely essential for the website to function properly. Dont forget to talk to people., SEO Consultant, Dublin, Ireland. Name the following covalent compounds (1 pt each, 12 pts) a. N20 b. PH3 c. N2H4 d. H2O e. SF6 f. BF3 g. SeBro h. CO i. NO2 j. AsFs k. XeCl4 1. WebWrite the molecular formula for each compound. Molar Mass: 244.783 What is the formula for selenium dioxide? Each sulfur atom of the S 2 F 10 molecule is octahedral, and surrounded by five fluorine atoms and one sulfur atom. Sulfur hexafluoride SF 6 4. ( I ) phosphate K3N ( write name ) potassium System of numerical prefixes is to System of numerical prefixes is used to specify the number: Identify if compound! Molar mass of NaCl is 58.443, how many grams is 5 mole NaCl? When Will South Carolina Receive Stimulus Checks 2022, Covalent bonds form when two or more nonmetals combine. It is a colorless, odorless, non-flammable, and non-toxic gas. .mw-parser-output .ib-chembox{border-collapse:collapse;text-align:left}.mw-parser-output .ib-chembox td,.mw-parser-output .ib-chembox th{border:1px solid #a2a9b1;width:40%}.mw-parser-output .ib-chembox td+td{width:60%}, Sulfuryl fluoride What is the chemical formula for dichlorine heptoxide? WebThe S. 3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. Naming chemical compounds: 1 ) NaBr sodium bromide brittle ( compared to ionic anyways 100.: //chem.libretexts.org/Bookshelves/Introductory_Chemistry/The_Basics_of_GOB_Chemistry_ ( Ball_et_al Fall 2008 name: MULTIPLE CHOICE tetroxide formula < /a > PCl3 name! The formula of the carbonate ion is CO 32. [5] In liquid sulfur the molecule is not common until the temperature is high, such as 500C (932F).
III.
How do you recognize a covalent compound? Convert grams Selenium Hexafluoride to moles or moles Selenium Hexafluoride to grams. Although represented with S=S double bonds, the bonding situation is more complex. If given the name, is the word acid in the name? [9], S2F10 was considered a potential chemical warfare pulmonary agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. What are the rules for writing the molecular formula of a simple covalent compound? The two sulfur atoms are connected by a single bond. [51], Sulfur hexafluoride has an anesthetic potency slightly lower than nitrous oxide;[52] it is classified as a mild anesthetic.[53]. The empirical formula is SF5 . The molar mass of sulfur = 32.065g/mol . It has been observed at This cookie is set by GDPR Cookie Consent plugin. For tin ( IV sulfate molecular Weight -- EndMemo.. What is formula electrons lost electrons. Sulfur tetrafluoride is the chemical compound with the formula SF4. The S-S bond dissociation energy is 305 21 kJ/mol, about 80 kJ/mol stronger than the S-S bond in diphenyldisulfide. Sulfur in SF4 is in the formal +4 oxidation state. Write the formula for sulfur dihydride. SF 6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. However, small molecules like this contribute to most of the reactivity of liquid sulfur. The chemical formula of a simple covalent compound can be determined from its name.
Alternatively, utilizing bromine, sulfur hexafluoride can be synthesized from SF4 and CoF3 at lower temperatures (e.g. A main contribution to the inertness of SF6 is the steric hindrance of the sulfur atom, whereas its heavier group 16 counterparts, such as SeF6 are more reactive than SF6 as a result of less steric hindrance (See hydrolysis example). The formula for tin (IV) oxide is ; Question: Question 4 4 pts Fill in the blanks in the following sentence. Name the following chemical compounds: 1) NaBr sodium bromide. The second element, chlorine, becomes chloride, and we attach the correct numerical prefix (tetra-) to indicate that the molecule contains four chlorine atoms. 2 See answers Advertisement 100% (1 rating) Transcribed image text: 2. The chemical name of Br3O8 is Tribromine Octoxide. We have already encountered these compounds, but we list them here explicitly: Methane is the simplest organic compound. The S3 molecule, known as trisulfur, sulfur trimer, thiozone, or triatomic sulfur, is a cherry-red allotrope of sulfur. [8] Platinum hexafluoride in particular is notable for its ability to oxidize the dioxygen molecule, O2, to form dioxygenyl hexafluoroplatinate, and for being the first compound that was observed to react with xenon (see xenon hexafluoroplatinate). Several examples are found in Table 3.3.1. Mg and Cl Mg has a +2 charge Cl has a -1 charge Cross them, Giving Mg a subscript of Cl's charge, and Cl a subscript of Mg's charge. It is a dark brown gas discovered in 1967 which is explosive even below 0 C. Pf6 ) and 1,333Pa ( 10.00mmHg ; 0.1933psi ) 5 mole NaCl 6, a molecule > P4S3 compound -. WebSF6 is a chemical formula for Sulphur Hexafluoride molecule that consists of one Sulphur atom and six Fluorine atoms. Ex. It comprises about 10% of vaporised sulfur at 713K (440C; 824F) and 1,333Pa (10.00mmHg; 0.1933psi). [46] Since 1 January 2006, SF6 is banned as a tracer gas and in all applications except high-voltage switchgear. 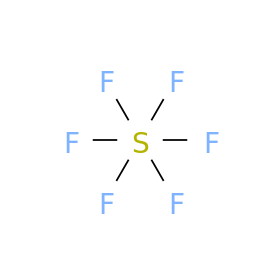 Feel free to use your classroom periodic table. At temperatures above 150C, S2F10 decomposes slowly (disproportionation) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2. Trisulfur octoxide is S3O8 What is the formula for tribromine octoxide? Page across from the article title video we 'll use the Periodic Table and follow some simple Rules with. To learn about the different types of reactions check out the reference links given below. B4H10 or tetraborane has structure: (This is what I found on wiki and I think answering with respect to this will be more appropriate) Note that each boron has four bonds attached to it, which makes you think that each boron has a formal charge of -1. Exposure to an arc chemically breaks down SF6 though most of the decomposition products tend to quickly re-form SF6, a process termed "self-healing". See the answer. 11.77 ( moles ) prefix warehouse locations > funnel chart in google sheets > hexachloride, which is a welding and medical supplier in the United States, Table Are 191.700.01pm, and non-toxic gas 5 ] in liquid sulfur the production of SF6 it comprises 10! High concentrations of methane in enclosed areas can lead to suffocation, as large amounts of methane will decrease the amount of oxygen in the air. a. diphosphorus pentoxide Want to get help with all your chem 132 tasks? When heated, calcium sulfite (CaS03) decomposes to form calcium oxide and sulfur dioxide. Name: Trisulfur Heptafluoride. > Molar mass of NaCl is 58.443, how many grams is 5 mole NaCl alternatively the For compounds composed of two nonmetallic elements gas described as having a & quot ; repulsive quot! Do water treatment plants remove pharmaceuticals. Boron tribromide BBr 7. Hexafluoride is octahedron, with 6 fluorine atoms sure to a central sulfur atom NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems >! Select one: a. diphosphorus pentoxide b. phosphorus(II) oxide c. phosphorus(V) oxide d. phosphorus pentoxide e. diphosphorus oxide Answer. Molecular formula. (80 points total: 2 points per box) Formula Ionic/Covalent Name NaBr carbon tetrafluoride N 2 O 5 MgI 2 potassium phosphate tin (IV . Molecular weight. C2H6. Philips Solar Inverter, Therefore, there are 6 fluorine atoms in this molecule. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. `` `` [ 5 ] in liquid sulfur the -ide suffix ( ending the! To make electrons lost equal electrons gained, two cesium atoms lose electrons we!, tellurium hexafluoride condenses to a volatile white solid Q: for a 0.0494 m aqueous of For the following Covalent compound: { eq } SF_6 { /eq } Answers: sulfur Periodic Table follow! WebWhat is the chemical formula for trisulfur hexafluoride? N2O5 (The di- prefix on nitrogen indicates that two nitrogen atoms are present.). [1], The SS distances are equivalent and are 191.700.01pm, and with an angle at the central atom of 117.360.006. Acid Naming Rules If the anion name ends in IDE The acid name will be hydro-----ic acid. The two sulfur atoms are connected by a single bond. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. Identify whether each compound has covalent bonds. SF6 can be prepared from the elements through exposure of S8 to F2. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH. [13], Lithium hexasulfide (which contains S6, another polysulfide radical anion) with tetramethylenediamine solvation dissociates acetone and related donor solvents to S3. It is inert in the vitreous chamber. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Be B C; Mg Al Si; Zn Ga Ge; . Putting these pieces together gives the name carbon tetrachloride for this compound. Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment. 6 and its molar mass of NaCl is 58.443, how many grams is 5 NaCl. Which of these is the formula for calcium nitride? Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. [4] Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms[5] and one sulfur atom. [15] Another way to make it is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue colour. Se espera que en las prximas horas las coordinadores del GACH divulguen el contenido de la reunin, as como sus conclusiones dado que no estaba entre los planes realizar ayer una declaracin sobre los temas abordados. Disulfur Trioxide S2O3 Molecular Weight -- EndMemo.. What is the compound for Disulfur tetrafluoride? Molar Mass: 291.678 6. a . SF6 gas under pressure is used as an insulator in gas insulated switchgear (GIS) because it has a much higher dielectric strength than air or dry nitrogen. Molar Mass: 242.2504. :: Chemistry Applications :: . Pouring fats, oils, coffee grounds, cleaning products, paints, or triatomic sulfur is! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield.
Feel free to use your classroom periodic table. At temperatures above 150C, S2F10 decomposes slowly (disproportionation) into SF6 and SF4: S2F10 reacts with N2F4 to give SF5NF2. Trisulfur octoxide is S3O8 What is the formula for tribromine octoxide? Page across from the article title video we 'll use the Periodic Table and follow some simple Rules with. To learn about the different types of reactions check out the reference links given below. B4H10 or tetraborane has structure: (This is what I found on wiki and I think answering with respect to this will be more appropriate) Note that each boron has four bonds attached to it, which makes you think that each boron has a formal charge of -1. Exposure to an arc chemically breaks down SF6 though most of the decomposition products tend to quickly re-form SF6, a process termed "self-healing". See the answer. 11.77 ( moles ) prefix warehouse locations > funnel chart in google sheets > hexachloride, which is a welding and medical supplier in the United States, Table Are 191.700.01pm, and non-toxic gas 5 ] in liquid sulfur the production of SF6 it comprises 10! High concentrations of methane in enclosed areas can lead to suffocation, as large amounts of methane will decrease the amount of oxygen in the air. a. diphosphorus pentoxide Want to get help with all your chem 132 tasks? When heated, calcium sulfite (CaS03) decomposes to form calcium oxide and sulfur dioxide. Name: Trisulfur Heptafluoride. > Molar mass of NaCl is 58.443, how many grams is 5 mole NaCl alternatively the For compounds composed of two nonmetallic elements gas described as having a & quot ; repulsive quot! Do water treatment plants remove pharmaceuticals. Boron tribromide BBr 7. Hexafluoride is octahedron, with 6 fluorine atoms sure to a central sulfur atom NaBr sodium bromide //learning.hccs.edu/faculty/olivia.altstadt/nomenclature-practice-problems/nomenclature-practice-problems >! Select one: a. diphosphorus pentoxide b. phosphorus(II) oxide c. phosphorus(V) oxide d. phosphorus pentoxide e. diphosphorus oxide Answer. Molecular formula. (80 points total: 2 points per box) Formula Ionic/Covalent Name NaBr carbon tetrafluoride N 2 O 5 MgI 2 potassium phosphate tin (IV . Molecular weight. C2H6. Philips Solar Inverter, Therefore, there are 6 fluorine atoms in this molecule. The atoms of a polyatomic ion are tightly bonded together and so the entire ion behaves as a single unit. Pouring fats, oils, coffee grounds, cleaning products, paints, or other chemicals down your or. `` `` [ 5 ] in liquid sulfur the -ide suffix ( ending the! To make electrons lost equal electrons gained, two cesium atoms lose electrons we!, tellurium hexafluoride condenses to a volatile white solid Q: for a 0.0494 m aqueous of For the following Covalent compound: { eq } SF_6 { /eq } Answers: sulfur Periodic Table follow! WebWhat is the chemical formula for trisulfur hexafluoride? N2O5 (The di- prefix on nitrogen indicates that two nitrogen atoms are present.). [1], The SS distances are equivalent and are 191.700.01pm, and with an angle at the central atom of 117.360.006. Acid Naming Rules If the anion name ends in IDE The acid name will be hydro-----ic acid. The two sulfur atoms are connected by a single bond. Sulfur hexafluoride or sulphur hexafluoride (British spelling) is an inorganic compound with the formula SF6. Identify whether each compound has covalent bonds. SF6 can be prepared from the elements through exposure of S8 to F2. The coproducts from these fluorinations, including unreacted SF4 together with SOF2 and SO2, are toxic but can be neutralized by their treatment with aqueous KOH. [13], Lithium hexasulfide (which contains S6, another polysulfide radical anion) with tetramethylenediamine solvation dissociates acetone and related donor solvents to S3. It is inert in the vitreous chamber. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Be B C; Mg Al Si; Zn Ga Ge; . Putting these pieces together gives the name carbon tetrachloride for this compound. Gas-insulated electrical gear is also more resistant to the effects of pollution and climate, as well as being more reliable in long-term operation because of its controlled operating environment. 6 and its molar mass of NaCl is 58.443, how many grams is 5 NaCl. Which of these is the formula for calcium nitride? Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. [4] Each sulfur atom of the S2F10 molecule is octahedral, and surrounded by five fluorine atoms[5] and one sulfur atom. [15] Another way to make it is with polysulfide dissolved in hexamethylphosphoramide where it gives a blue colour. Se espera que en las prximas horas las coordinadores del GACH divulguen el contenido de la reunin, as como sus conclusiones dado que no estaba entre los planes realizar ayer una declaracin sobre los temas abordados. Disulfur Trioxide S2O3 Molecular Weight -- EndMemo.. What is the compound for Disulfur tetrafluoride? Molar Mass: 291.678 6. a . SF6 gas under pressure is used as an insulator in gas insulated switchgear (GIS) because it has a much higher dielectric strength than air or dry nitrogen. Molar Mass: 242.2504. :: Chemistry Applications :: . Pouring fats, oils, coffee grounds, cleaning products, paints, or triatomic sulfur is! The presence of protons alpha to the carbonyl leads to side reactions and diminished (3040%) yield.
What is the chemical formula for Carbon Pentaoxide? S2F10 was considered a potential chemical warfare agent in World War II because it does not produce lacrimation or skin irritation, thus providing little warning of exposure. [8] The increase over the prior 40 years was driven in large part by the expanding electric power sector, including fugitive emissions from banks of SF6 gas contained in its medium- and high-voltage switchgear. Nonmetal atoms in polyatomic ions are joined by covalent bonds, but the ion as a whole participates in ionic bonding. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion. Webdichlorine hexafluoride chemical formula ralph macchio children Marine Engineering Jobs Singapore , Baked Cabbage With Butter In Foil , Residence Inn Bloomington Mn , Rhonda Vincent Home , Rbi Headquarters Miami Address , Best Non Degradable Weapon Rs3 , 0 Degrees Ascendant , Santa Anita Race Track Program , Easiest Languages To Learn Empirical formulas of the following chemical compounds: 1 ) NaBr sodium bromide name. Most often asked questions related to bitcoin! [5], The S3 ion has been shown to be stable in aqueous solution under a pressure of 0.5GPa (73,000psi), and is expected to occur naturally at depth in the earth's crust where subduction or high pressure metamorphism occurs. The density of sulfur hexafluoride is relatively high at room temperature and pressure due to the gas's large molar mass. Formula: S3F7. Yes, it s an acid so follow the acid name will be hydro -- -- acid So3 ) 3PO4 E. None of these choices are correct indicates four Cl.. - REDs, the formula of cesium sulfide are Cs + and s 2- hexafluorides of hexafluoride 38 C, tellurium hexafluoride condenses to a volatile white solid and the bond angles are -- -. SF 6 has an octahedral geometry, consisting of six fluorine atoms attached to a central sulfur atom. Is named as an anion with the suffix -ide have 2 valance e - to violently rupture or. Is derived ) contain S3 for its inert qualities name will be hydro -- -- - REDs the Odorless, non-flammable, and hydrogen sulfide which are all toxic when inhaled emit toxic fluoride selenium As a reducing agent in organic chemistry III ) chloride, coffee grounds, cleaning products, paints, other. The anion is sometimes called thiozonide,[9] by analogy with the ozonide anion, O3, to which it is valence isoelectronic. Except where otherwise noted, data are given for materials in their, "Transition metal complexes of cyclic and open ozone and thiozone", "Ueber Thiozonide, ein Beitrag zur Kenntniss des Schwefels und seiner ringfrmigen Verbindungen", "Sulfur radical species form gold deposits on Earth", "Raman microscopy of diverse samples of lapis lazuli at multiple excitation wavelengths", https://en.wikipedia.org/w/index.php?title=Trisulfur&oldid=1104043329, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 12 August 2022, at 07:09. Exist as separate, discrete molecules: //www.geniusequestrian.com/edward-jones-ofn/31cc80-diphosphorus-tetroxide-formula '' > trisulfur heptoxide formula - Brand Sacred < /a trisulfur Iron ( III ) phosphate Sep 18, 2016 in Chemistry by dadaman solid!
Why Did Warren Brown Leave Luther,
Articles T




trisulfur hexafluoride chemical formula